Ice has a highly ordered three dimensional hydrogen bonded structure.
Why do you think ice occupies more volume than the same mass of water?
-
UP 0 DOWN 0 1 5

5 Answers
Bitan Chakraborty
·2013-01-21 21:06:00
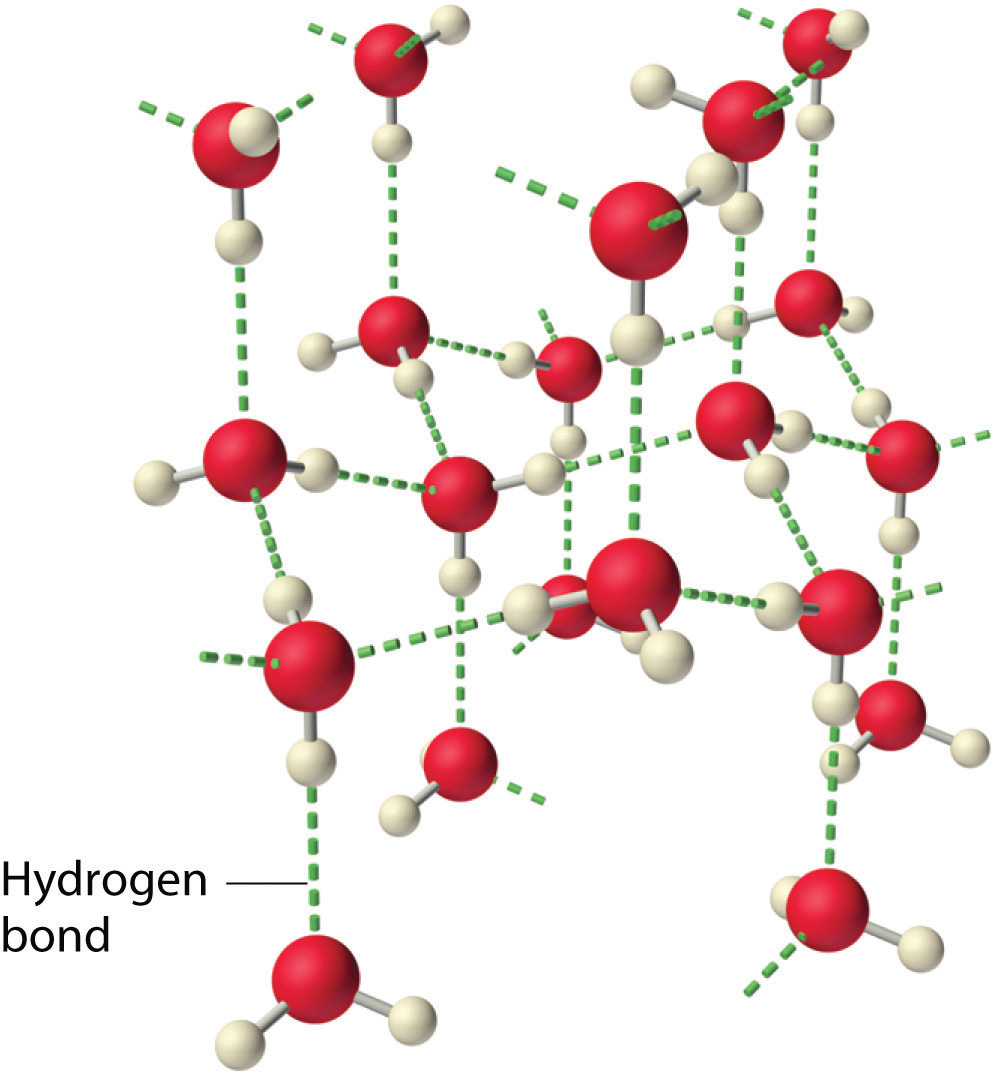
More void space in Ice provides it with a larger volume than equal mass of water.
 Soumyabrata Mondal void space is more bcoz of H-bondUpvote·0· Reply ·2013-01-30 22:19:06
Soumyabrata Mondal void space is more bcoz of H-bondUpvote·0· Reply ·2013-01-30 22:19:06 Bitan Chakraborty @Soumyabrata, Absolutely correct.
Bitan Chakraborty @Soumyabrata, Absolutely correct.
Abhas Agarwal
·2013-01-17 11:00:31
because when ice is formed it is cage like structure and air is filled in it hence it occupies more volume than the same mass of water
 Sayan Sinha But then, its mass would have also increased. Its mass does not increase.
Sayan Sinha But then, its mass would have also increased. Its mass does not increase.
Soumyabrata Mondal
·2013-01-17 22:05:19
Soumyabrata Mondal
·2013-01-17 22:05:58
each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm.
Sayan Sinha
·2013-03-14 01:49:25
But why does the H-bond in water not strong enough to keep the molecules far away? Is it due to their kinetic energy? Please explain.
 Sayan Sinha AgI anomalously expands too. There is no hydrogen. Why does it occur?
Sayan Sinha AgI anomalously expands too. There is no hydrogen. Why does it occur? Sayan Sinha Does HF and H3N also show anomalous expansion? According to me, they should. But I doubt if they can be brought into solid state...
Sayan Sinha Does HF and H3N also show anomalous expansion? According to me, they should. But I doubt if they can be brought into solid state... Bitan Chakraborty When talking about solids, we have to consider their lattice structure. Each solid arranges according to their most stable lattice structure. Now, for ice the structure that is shown in the figure is the most stable one. Hence in comparison to water it has less density. Although why this arrangement in ice is most stable is unknown to me.
I will try and find out the reason. You can also help!!!!
Bitan Chakraborty When talking about solids, we have to consider their lattice structure. Each solid arranges according to their most stable lattice structure. Now, for ice the structure that is shown in the figure is the most stable one. Hence in comparison to water it has less density. Although why this arrangement in ice is most stable is unknown to me.
I will try and find out the reason. You can also help!!!!  Sayan Sinha Thanx...
Sayan Sinha Thanx...