Thanks Correct ans A
What is the decreasing order of strength of the bases 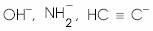 and
and 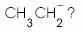
(a) 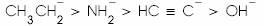
(b) 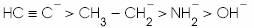
(c) 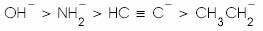
(d) 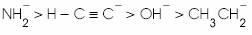
-
UP 0 DOWN 0 0 3

3 Answers
Pritish Chakraborty
·2010-04-14 10:32:45
Add a proton to compare the strength of their conjugate acids.
The one least likely to lose proton is the alkane. So its conjugate base is highly basic.
Ammonia is a very weak acid. When it loses proton it becomes unstable. So its conjugate base is second in order of basicity.
Ethyne is quite acidic. It can easily lose its proton due to the sp hybridisation of the triple bond. Hence it is weakly basic.
Water is also a weak acid.
Order should be C2H5- > NH2- > HC≡C- > OH-...
Alkanide ions are very unstable(such as ethanide, here, so it quickly accepts proton, making it a very strong base).