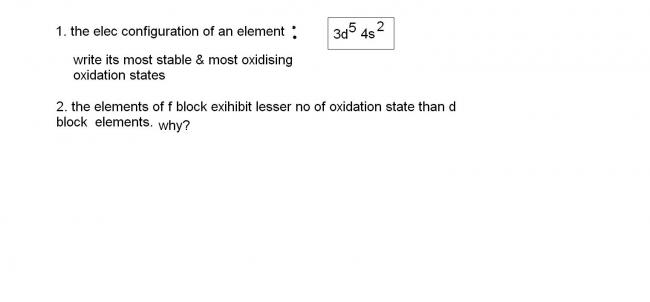
the element is Mn..which has most stable oxidation state as +2....and most oxidising oxidation state as +7...
ans 2...the 4f electrons are effectively shielded by 5s and 5p electrons so that they cannot be easily lose easily..so the lanthanide elements do not exhibit a variety of oxidation states....