What is the effect of pressure on melting pt of ice?
Shouldnt an increase in pressure increase the melting point?
having a great controversy over this!plz answer.
-
UP 0 DOWN 0 0 16

16 Answers
increase in pressure involving change in mp of ice...[3][3]
well i'll try to explain this by two methods...
first is physics method...
the general explanation....[1]
but first i'll ask a question..
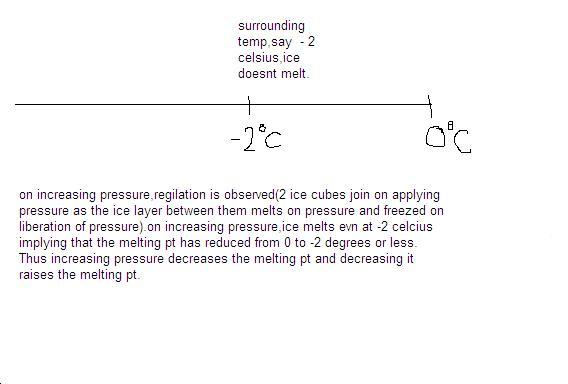
when we press together two different blocks of ice together at const temp of 0°C, first a few drops of water come out and then on releasing the pressure, the two blocks get welded to form one block...why is it so??
i'll nt w8 4 answer since nobody is onl9...i'll myself post answers now..[1][2][3][1][2][3]
answer is when we press two blocks of ice tgether, the pressure increasing decreases the m.p. thus at even 0°C...or -1°C or -2°C the ice melts....since now mp is much below the temperature....
then again when we leave the pressure, the mp increases back to 0°C and the water formed between the two ice blocks again solidifies thus welding the two ice blocks together...[1][1]
this was the example of increasing pressure decreasing the m.p.
thus when we decrease pressure, the m.p. must increase...[1][1][1]...
reason no. 2
the atgs reason...[3][3]
water(solid) water(liquid)
(larger volume) (less volume)
let the above conversion be in dynamic equilibrium at 0°C
on decreasing the pressure, by le chatelier's principle, the equilibrium shifts towards left...
thus at 0°C more ice is formed....to melt which the temp must be risen...thus lowering the pressure increases m.p.
well to be frank this is one of my useful whims...may be this is absolutely wrong explanation but this proves helpful to remember things at examination hall....i'll post some more useful whims when relevant topics come up...[1][1]
after thinking much on the topic i think i have got the exact physical interpretation of the thing...[1][1][1]
The actual reason...
the inter particular spaces in ice is more than that in water...
thus decreasing the pressure, the ice molecules experience an outward expansion, which helps the ice to increase the volume...thus to reach the above state, its temp must be risen...also after reaching the original state at elevated temperature, the m.p. obviously increases...
i dont care abt the other two reasons but please comment if the 3rd reason is correct please...[4]
then where was the confusion?? it was so crystal clear...isnt it???[1][1][1]
actually i was confused earlier myself in another post.
but got it on thinking coolly[1]