@pranav....plz give ur reasoning.
@arka...no prob buddy, usi bahaane apan ka dimaag toh chala :P
Yet to be Answered/Explained/Confirmed - Qn(s) 1, 6, 9, 10.
Good Obj. Qn(s)...
Needed to clarify & verify my answers, 'coz i don't have the list of correct ones.
SINGLE ANSWER CORRECT

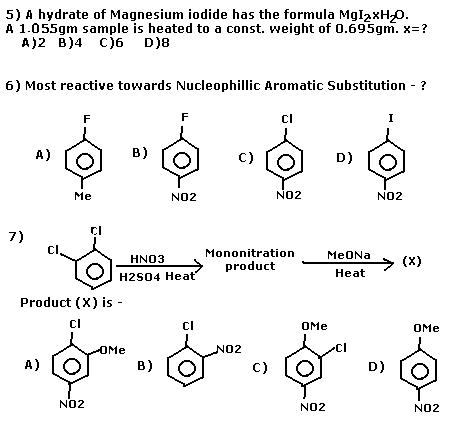
MULTIPLE ANSWERS CORRECT
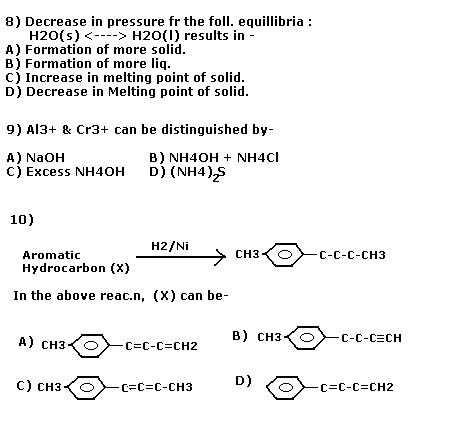
Do post the answers u all got.
@pranav....plz give ur reasoning.
@arka...no prob buddy, usi bahaane apan ka dimaag toh chala :P
Yet to be Answered/Explained/Confirmed - Qn(s) 1, 6, 9, 10.
Nah, i still persist....
Abhi jis state mein ice n water were in equilibrium, the temp. must have been at least 0 degree (the original melting point)....maan lo 2 degree par hain.
Aur agar solidify hone ki tendency is increasing toh iska matlab ki the gap b/w the actual temp. & so-called M Pt. shud Decrease......aur actual temp. toh change ho nahi rahaa, so, the only way 2 do tht is increasing the melting point.
It is smwht similar to if i ask, 5 degree par zyaada paani milega ya 15 degree par...?
check it avik...
tendecy to melt is decreasing, agreed
so the melting point decreases.
if the melting point had increased,as according to u,it would have melted relatively easily at a given temperature.
So,a,d are correct!
Arrey arka...
Lowering the pressure will take the reac.n 2wards higher volume i.e. 2wards the form.n of ice....
tht means more n more ice is being formed & its tendency 2 melt is decreasing....Hence, the melting point is Increasing. So A,C theek hain.
@avinav... Clemmenson is a reagent with an acid solvent, toh woh acid required kaam karne ke bajaye -OCH3 se hi chipak jayega...n we won't get the desired product. Hence (A) Wrong.
guyz..for Q3. i somway feel..its (a)..can any1 explain the ans givn abov
2) See my approach-
a =2√2*r
Edge length occupied = 2r.
Fraction Req. = (2√2*r -2r)/(2√2*r) =0.29
Yeah, B) ...tht was a typo in #3
5) D...Agreed.
7) C...Agreed.
8) A,C...Agreed too.
10) Qn mein kuch ghapla lagta hai...Read #5.
Q7. (C) as the Cl o/p to the nitro grp will be substituted while the one m to the nitro grp will remain as it is
2) c
3) b
4) c
5) write the molar wt of the compund as weight of Mg + 2(wt of I) + x(wt of water), then frm the info given, calc no. of moles of water lost...that will be x ((not in a mood to calculate myself [3]))
6) d
10) most probably a, c
Hey how u got C for (2) guys..
have u done (1-PF)=1-0.74=0.26
they have mentioned 'edge'..
I think ans is (B).
6) Nearly same as Q6 here-
http://targetiit.com/iit-jee-forum/posts/aromatic-doubts-13471.html
Not clear though.
Arrey! i wasn't taking the von't hoff factor only...[3]
Thnx fr tht.[1]
What a blunder ! OMG!
4) 0.2 mol of AgCl will be precipitated. so, 0.2 mol AgNO3 will remain in soln. therefore, molality = 0.2/6 (assuming density of soln= density of pure water)
m=1/30
ΔT= i Kf m
and 1 mol AgNO3 ≡ 1Ag + 1NO3
=> i =2
putting the values, we get ΔT = 0.124
10) How can v have D) ? Woh methyl Group kahaan se aayega ?? Moreover, 3 of the compounds given are non-planar, so how can they be Aromatic ?
6) bcz size of F matches that of C...so better resonance and stronger bond. since I is much larger than C, C-I bond is weakest. and then NO2 creates the nucleophilic attack site
Theek hai, got C) too fr 2)...
3) also B)...ok
4)My answer wasn't matching ny of them...post the working na plz.
5) Yep, got 8, wanted 2 confirm.
6) why D) ??