ya me too thought the same manmay.strangely a was given as the answer.
ADDED14.which of the following halogen acids is least basic
a)HF b)HCl c)HBr d)HI
ans given a
shouldnt HI be the least basic(most acidic) among these due to the greater bond length of HI bond?plz clarify.
15.ortho and para H2 differ in
a)nuclear charge b)nuclear reaction c)electron spin d)proton spin
-
UP 0 DOWN 0 0 27

27 Answers
oh yeah,it was very obvious indeed rickde.but then in that question,the energy due to thermal motion should also refer to the energy on account of vibrational motion shouldnt it?
Q16. Without a doubt the alcohol is the strongest acid. Oxygen is a electronegative group than is C≡C. Methanol's conjugate base is able to accommodate the negative charge better than C≡C does.
translational motion of a molecule is given by 3RT/2 and this holds irrespective of whether the gas is monoatomic or not".
each degree of freedom has 1/2 RT energy associated wit it.....
for translational motion...there r three degrees (x, y, z) irrespective of the nature of molecules of gas.... hence translational energy is 3RT/2.....
the total energies r different as rotational n vibrational degrees r different
Q 16> CH3OH
i guess u r confused between alc and alkyne ,
consider the conjugate bases , negative charge will be preferred on oxygen that that on carbon , so conj base of methanol is more stable than that of acetylene , hence the reason.
Q 14> dude , most acidic doesnt mean least basic ,
if it was that way , then i could say that acetic acid is a strong base since it is a weak acid, wich aint true
i think HF will be weakest base , since the conjugate acid will be bearing a +ve charge of F, wich is comparitively less preferred than a +ve charge on other halogens
hence it will be least basic
Fresh doubts reagrding thermo question(number 21)
as i had thought and as avirup said in #19 that U=5RT/2 from which we proceeded and didnt get the answer.
Now in quantum physics from Berkley phy course,it is stated:
"The avg kinetic energy associated with translational motion of a molecule is given by 3RT/2 and this holds irrespective of whether the gas is monoatomic or not".
how is this possible,the explanation isnt given there in the book..
someone plz elaborate if possible.
thanks a lot archana and sandipan.
abhirup i'm still not convinced regarding that number of moles.
if the vol is 0.25 m3(250 L),then certainly its not 1 mole(1 mole wud occupy 22.4 L)
for 20
M(median) = \left\{L+\frac{\frac{N}{2}-c}{f}xh\right\}
where...
L=lower calss limit of the median class
f = frequency of the median class
h = width of the median class
c= cumulative frequency of the class just preceding the median class
N = \sum{f_i}
source=R.SAgarwal class 11 Pg. SP-7
also a discussion on this topic is at http://www.tutornext.com/mean-mode-median-grouped-data/1983
-------------------------------------------------------------------------------------------
var = \frac{\sum{x_{i}^{2}}}{n} - x 2
so \frac{\sum{x_{i}^{2}}}{n} = n ( n + 1 ) ( 2n + 1 )6n = (n+1)(2n+1)/6
and x 2 = \left( \frac{n(n+1)}{2n}\right)^{2} = (n+1)2/4
so varience of n natural nos. = (n+1)(2n+1)/6 - (n+1)2/4
------------------------------------------------------------------------------------------
to find the variance of suppose a,b and c
first find x=(a+b+c)/3 (i.e the mean of the nos)
then find ((a-x)2+(b-x)2+(c-x)2)/3 which actually variance
now aplly it for natural nos.:)
U=FRT2 where F=degrees of freedom.
For diatomic, F=5
So, U=5RT2=5PV2.... now the answer will come which is (b)
my question is shouldnt the internal energy be 5RT/2?
how can number of moles be equal to 1 here,so that RT=PV???
the mass given is 1 kg.so for n to be equal to 1,the molar mass of the gas has to 1000which is probably not possible!
acc to the solution provided,
thermal energy corresponds to the internal energy.
volume=mass/density=1/4
internal energy of diatomic gas=5PV/2 =5*104
21) 1 kg of a diatomic gas is at pressure 8*104Nm-2.density is 4 kg/m3.wats its energy due 2 thermal motion
a)3*104 b)5*104 c)6*104 d)7*104
answer to 18 shuld have been (d)
assuming complete dissociation i.e, x = 1
K3[Fe(CN)6] → 3K+ + Fe(CN)63-
here i = 1 + ( y-1 ) x where y is no. of products hence here i = 1 + ( 4 - 1 ).1 = 4
and in case of Al(NO3)3
we have Al(NO3)3 → Al 3+ + 3 NO3- here too y = 4 hence
i = 1 + (y - 1)x → i = 1 + (4 - 1).1 =4
hence it seems to me that (d) has same vant hoff's factor as K3[Fe(CN)6]
20.plz tell me the way to find the median for a class without using an ogivehad earlier done so using an ogive.
also how to find variance of n natural nos.
or provide a link on these
For Q.19>
+ ve deviation is shown by (d) bcoz..the H-bonding of methanol is affected after the addition of benzene--->escaping tendency into vapor pahse increases ---> VP increases
for this type of qs..its very confusing to make out the answer....a quick recap of the this part before exam is often need
the ans given is d for 19.
options a,c represent -ve deviation(maximum boiling azeotropes).
is the reason d as H bonding is hindered decreasing interactions between them and thus more of the solvents move in vapour phase??
plz tel me if i am wrong.
ratne hi padhte hain woh,5-6 example hote hain
koi aake bolega usne samjha hai,lekin I bet agar naya kuch aaya toh woh nahi bata sakta isme
thnaks akshay.sum1 plz try 19.is there any way to determine the ans of 19 loically or are these to be just rememberedwhich is impossibl!
16 Reason
always for checking acidic strength first check group
always COOH>OH>C H>
H>
16
d
17
d
Reason:In a the formed carbocation would be very unstable
In b it would be stable but less than c and d
In c and d d has more resonance
18 d
More doubts:
16)among the foll compounds,strongest acid is
a)C2H2 b)C6H6 c)C2H6 d)CH3OH
17)dehydration is most in:(reason too)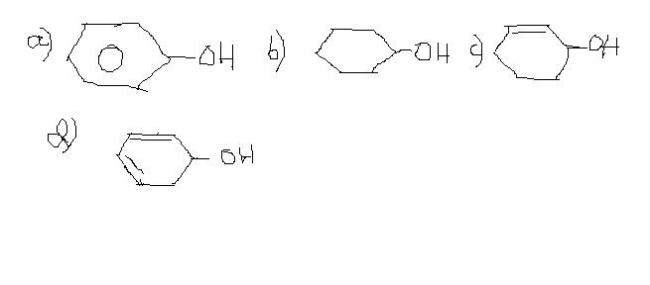
18.which has the same vant hoff factor as K3[Fe(CN)6]
a)Al2(SO4)3 b)NaCl c)Na2SO4 d)Al(NO3)3 i think d ans given a
19)which of the following show +ve deviation
a)water-nitric acid b)acetone-chloroform c)water-HCl d)benzene-methanol
ya both of u seem right to me.
although ans to 14 is given as HF which shud be wrong i think.
1..d
2.Molecular hydrogen occurs in two isomeric forms, one with its two proton spins aligned parallel (orthohydrogen), the other with its two proton spins aligned antiparallel (parahydrogen)
so d