Edit : Why can't it have? Chalo, +3 state is most stable due to noble gas config, then it(Sc) shouldn't be obtained in any other state. That it occurs in +2 state means it exhibits variable oxidation states. But because +3 gives noble gas config, it exhibits variable states to a much smaller extent.
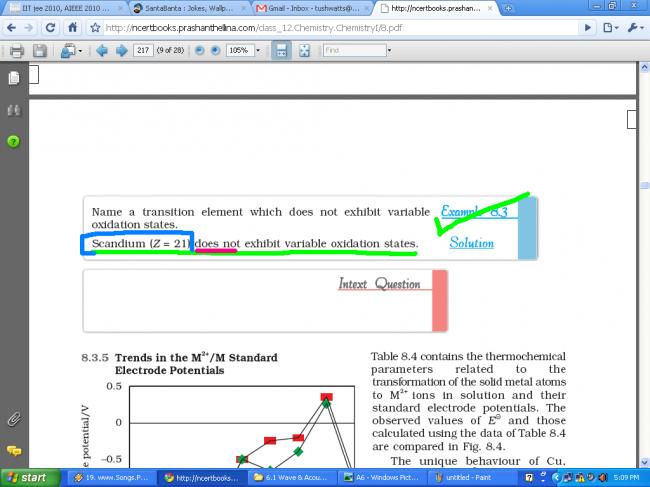
Scandium (Z = 21) shows variable oxidation states or not ???
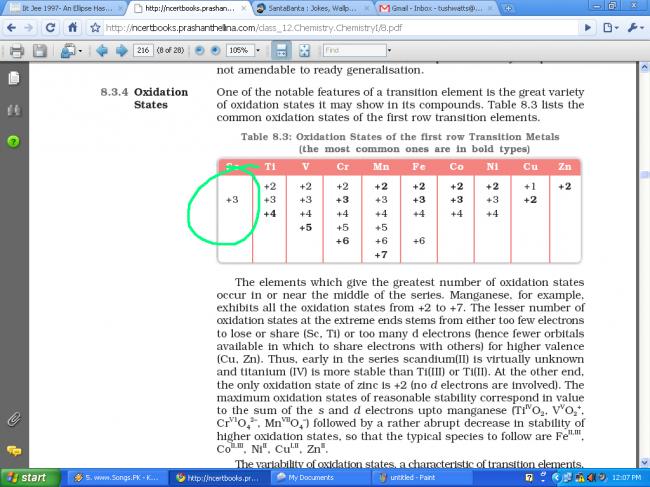
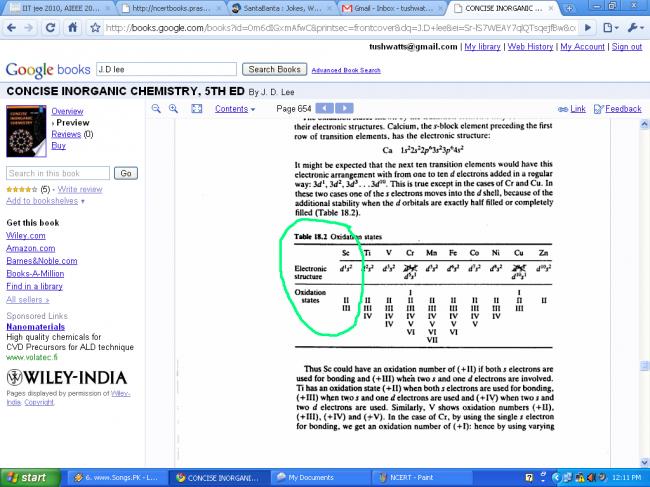
NCERT says NO, but Brett Lee says YES.
-
UP 0 DOWN 0 1 5

5 Answers
Pritish Chakraborty
·2010-03-21 00:49:32
Tapas Gandhi
·2010-03-21 04:59:39
most common oxidising state is +3
BUT

The scandium in scandium dihydride formally is in the oxidation state 2.
Pritish Chakraborty
·2010-03-21 05:18:54
Tushar, you need to only look at your TiiT signature to see what I mean :P
+3 state for scandium is most common, but as tapas showed, it can have formal oxidation state of +2. Because it acquires noble gas config in +3 state, it does not exhibit that many variable oxidation states as the other transition elements do. Mushkil se +2 dikha sakta hai.
It's the same reason zinc prefers +2 state.