C
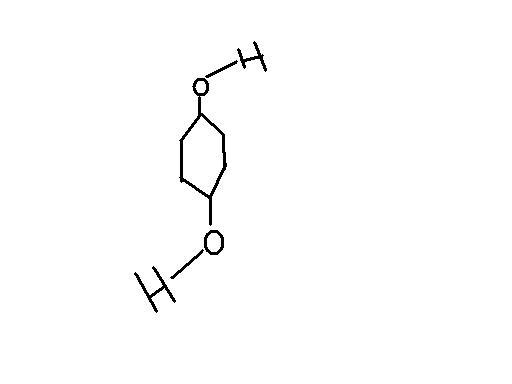
Which of these have non-zero Dipole moment??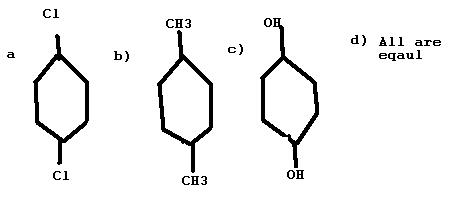
with proper explanation!!

quinol exhibits a non leniar structure.that's the reason for it exhibiting a non zero dipole movement
i got a doubt!!
if suppose in dicholoro benzene,
both the chlorine are above the plane or below the plane then what?
will μ be =0??
i dont think so...
what do you guys think?
cyclo hexane is in NO-WAY a planar molecule...
instead there are many non-linear conformations and thats the reason why we have isomers called chair boat half boat twisted chair and all...
in the most stable (lowest potential energy) conformer that is chair conformer has the opposite carbon protruding in opposite directions in plane made by the other four carbon atoms...
so in the chair conformer,
all mono membered groups must exhibit zero dipole moment!!!
O-H exhibits non-zero dipole moment due to the O-H bond not the C-O bond!!
my present thoughts now contradicts my previous one...
well, if we fix the chair conformer only,
the 2 O-H bond will also have many conformations one of which will be of trans nature and another of cis nature...
actually the conformations will be way more complex than 2-d cis-trans...there will be 9 conformations in 3-d equivalent to cis-trans conformation in 2-d
this will ensure zero dipole moment even for the quinol structure....
so answer i think will be (d)
subhomoy in fiitjee's material this question is there...
answer given c..
logic as told earlier by people...
here answer comes out to be c because we assume everything is on a plane
my point is what if i say they are on different planes...i mean both cl above the plane...
like in this case
here both are above the plane... so net dipole momnet gets added...
if one is above the plane and other is below...
then μ gets nullified....here
we cant jump into any conclusions directly...can we??
subho tera explanation ka dy/dx=0 ho gaya...:P:P
dont know about fiitjee..
was telling whatever i had read in Morrison Boyd quite some time ago now...
i am quite sure that
1) the molecule cannot be planar
2) there are 9 enentiomers involving O-H
3) out of these 9 only 3 provides dipole moment
4) resultant dipole moment though cannot be neglected will be very very less...
5)this is only for the chair conformer...
6) like this there will be many more enantiomers involving the chain of carbon...(1,4-diol)...
yea true the answer should have been c not d
dont know what mede me write d then... :P
arrey subho i also know that!!!
now tell me about my doubt post previous yours!!
no 11.
uska kya??
well look
since the hexane ring doesnt have planar structure, on the positions specfied, one forms the more crowded position and the other the free position (i am again talking about chair conformer)...
and by the design of chair conformer (:P) the less crowded areas on the 1,4 positions lie in opposite to the plane...
and if u know, Chlorine is much larger an atom than hydrogen, thus, it tends to occupy the more free position and thus it is not a sheer coincidence that the groups appear as one above and the other below the plane...
the reason of such existence is to minimize steric hindrance....and is achieved by rotation about single bonds which we know is the most easy rotation...
thus the logic what fiitjee has given is correct from here on...
i am giving a diagram of what i am intending to say...
that will help u to understand better....

the circled positions are the less hndered positions occupied by chlorine...
and by the shape they are present on the either side of the (approximately) planar structure of cyclo-hexane ring....