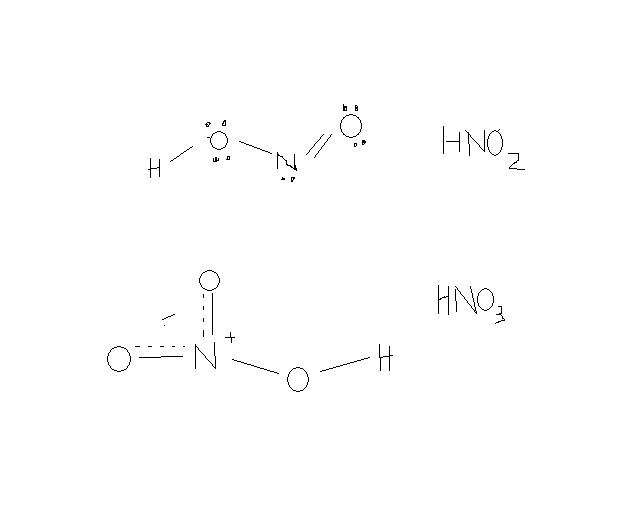
in HNO3, there is resonance... so we can say that it has two partial double bonds connecting N and O...
whereas in HNO2, there is only one..
thus statement 2 is correct..
st1: hno3 is strongr acid than hno2.'
st2: in hno3, there are 2 nitrogen to oxgen bonds, but in hno2,there's only 1.
in ans--st 2 is given correct....
pls confrm it shud be incorrect.
thanx

in HNO3, there is resonance... so we can say that it has two partial double bonds connecting N and O...
whereas in HNO2, there is only one..
thus statement 2 is correct..
but N to O bonds are 3 in hno3, aren't they?..why did u count only double bonds?
in HNO3,
THERE ARE 2 nitrogen to oxygen bond (bonded directly.)
the other one is a N-OH bond not a N-O bond.
obviously st-1 is correct and st-2 is false. there are 3 N-O bonds in HNO3 . some day pour HNO3 and HNO2 on yr different hands. u would get the feeling within a moment. the Ka value of HNO3 is very high- about 103.
and this question was an assertion reasoning one.