As a ligand approaches the metal ion, the electrons from the ligand will be closer to some of the d-orbitals and farther away from others causing a loss of degeneracy.See if ligand approaches in an axial direction they will encounter first the electron cloud of d(x2-y2) and d(z2) orbital.so the replulsion causes increase in energy as compared to that in d(xy),d(xz),d(yz) orbital.see figure 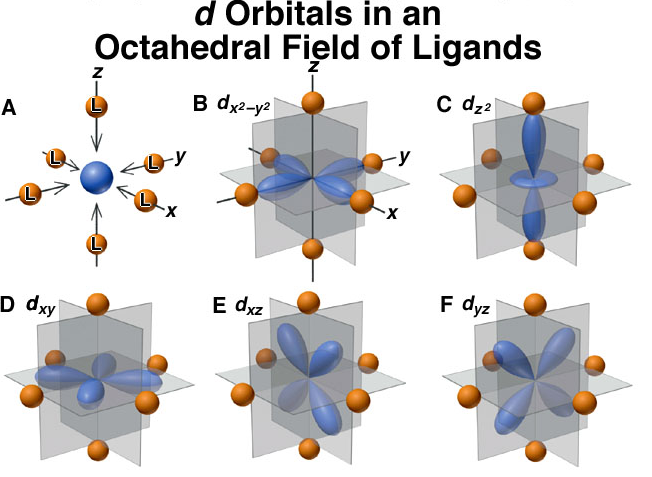
surrounding groups affect the energy of some of d orbital more than the others. thus, the d orbtals are no longer degenerate- why should surrounding groups affect the energy of some d orbital more than the others when they r previously degenerate??
-
UP 0 DOWN 0 0 1

1 Answers
Manish Pankaj
·2014-06-24 03:30:26