Nitrogen doesn't exhibit +5 state...it is for exactly that reason(absence of d-orbital). Where did you see it show +5 state?
Maximum covalency 4 meaning along with the lone pair, nitrogen is capable of forming 4 bonds, such as in R4N+.
im having a very basic doubt regarding "oxidation state".how can nitrogen have +5 oxidation state when it does not have a d-orbital??is it neccessary to have n "unpaired" electrons to show +n ox. state?and how is its maximum covalency 4?for covalency4 it should form 4 covalent bonds using 3p and 1s ?but in s two electrons are paired.how can those form a bond??confused..
-
UP 0 DOWN 0 0 6

6 Answers
"absence of d-orbital" ....for all elements on earth infinite no of orbitals r present...its just that they r not filed wit electrons
@pritish NCERT says so..nitrogen has +5 oxidation state in N2O5
Oh wait..I got confused myself. It does show +5 state, sorry.
I'll have to reread p-block...forgotten everything.
it is a coordinate bond.. hence it can show +5 state.
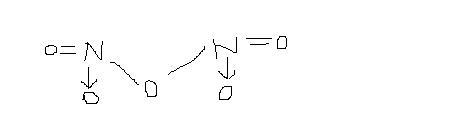
coordinate bond can be replaced by = (double bond) but it is not actually double bond. So from the structure it is evident that N can show +5 state.. The lone pair is used in electron-pair donation