help
15 Answers
Akand
·2009-03-31 00:18:38
i dont think its polar...... unless it dimerizes and make a very complex structure...................
or else para-dihydroxybenzene isnt polar i guess
ДвҥїÑuÏ now in medical c
·2009-03-31 00:26:51
yup...it is not linear....so dere is dipole moment
big looser .........
·2009-03-31 00:27:58
Akand it is given that it is polar and they had asked the reason why dichlorobenzene is non polar while dihydroxybenzene is polar
ДвҥїÑuÏ now in medical c
·2009-03-31 00:34:24
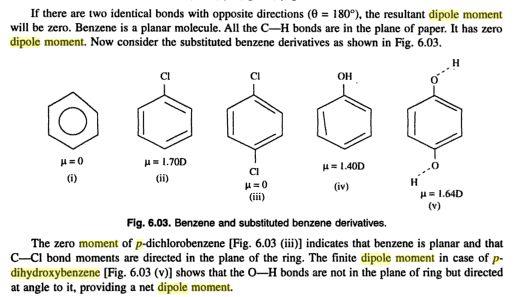
copied from An Introduction to Physical Chemistry By Das Ishwar, Archana Sharma, Namita Rani Agrawal
greatvishal swami
·2009-03-31 00:43:09
yup abhi is rite its polar and the reason is the same given by him
