That;s an easy one lubu...the structure is given in NCERT ..there is no S-S linkage..
Number of S-S bond present in P4S3 is ___.
Can we do this if we dont know the structure ?
-
UP 0 DOWN 0 0 6

6 Answers
Avik
·2010-04-06 12:11:46
Won't imagine to attempt in such a situation,,,
Because...it won't click so easily....because...there are "Zero" S-S bonds...!!!
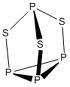
govind
·2010-04-06 12:19:35
Asish Mahapatra
·2010-04-06 22:31:20
waht abt P4S7 ?
ques: AIATS 2011
No. of P-S-P bonds is x and P-P bonds is y in P4S7
then x+y =
utd4ever
·2010-04-06 23:43:12
S3O9 is a trimer of SO3 it has three S-O-S bonds but no S-S bonds ....