 1
1A 0.81g sample of pyrolusite ore(impure MnO2) is treated with
1.651 g of oxalic acid (H2C2O4.H2O)
in an acidic medium.Following this reaction the excess oxalic acid is
treated with 0.1M KMNO4 , 30.6 ml being required.What is
the % of MNO2 IN the ore
H2C2O4 + MnO2 + 2H+ → Mn2+ + 2H2O + 2CO2
5 H2C2O4 + 2 MnO4- + 6 H+ → 2 Mn2+ + 8 H2O + 10 CO2
 1
1for post no.26----------------sodium and potassium
 1
1ans to the [Zn+2] question should be (c) according to le-chatlier principle
 1
1ans should be (a ) fecl3/dil.hcl .blood red due to formation of fe(cns)3
 1
1Nitrite + acetic acid + thiourea → N2+SCN-+2H2O
Formation of the product in the above reaction can be identified by:
(a) FeCl3/dil.HCl when blood red colour appears
(b) FeCl3/dil.HCl when blue colour appears
(c)K2Cr2O7/dil.HCl when green colour appears
(d) KMnO4/Hcl when colourless sol is formed.
 1
1The reaction
4MO2(S) + 2CO2(g) → 2M2CO3(s) + 3O2(g)
is to be used to regenarate oxygen in space-craft.
Can anyone tll me the choice of M among alkali metals...
 1
1In strongly alkaline sol containing excess of barium ions,a sol containing
0.01587g of I- was treated with o.1 molar MnO4-
until a pink colour persisted in the sol:
10.0ml of the MnO4- sol was required.under these
conditions MnO4- is converted into the sparingly soluble
BaMnO4.What is the product of the oxidation of iodide?
 1
1Last one :
A - NH4NO3
B - H2O
C - N2O
D - P4O10
HINT : only oxide liquid at room temp. possible is perhaps H2O. that stong dehydrating agent struck for P4O10. AND finally dat 2500C data struck for NH4NO2.
FOR PREVIOUS ONE its perhaps HCl (aq.).
 1
1A colourless inorganic salt (A) decomposes completely at abt 250
celsisus to give onli 2 prodcts (B) and (c) leaving no residue.The
oxide (c) is a liquid at room temp and neutral to moist litmus
paper whil the gas (B) is neutral oxide.White phosphorus burns
in excess of (B) to poduce a strong white dehydrating
agent(D). Write eqs for the reactions involved in the process..
 1
1To increase significantly the conc of free Zn2+ion in a sol of the
complx ion [Zn(NH3)4]2+,
Zn2+(aq)+ 4 NH3 (aq) → [Zn(NH3)4]2+(aq)
add to the sol some:
(A) H2O
(b) HCl(aq)
(c) NH3(aq)
(D) NH4Cl(aq)
 1
1not sure, but perhaps c). seen somewhere.
 1
1dude,philosopher's wool is ZnO (zinc oxide)
thus answer is b) CoZnO2.
the other one i'll post tomorrow.
 1
1Philosopher's wool on treatment with cobalt nitrate produces
(a) CoBaO2
(b) CoZnO2
(c) CoSrO2
(d) CoMgO2
 39
39accord to me `a` is the right answer.
as its 5 water molecules associated with it. and it melts at 30 degree celcius (which is room temp generally)
 1
1the Answer is obviously (a) because NaOH is not Deliquesent and SiO2 is sand
You can test it to BE deliquesent by just seeing it in a plant
 1
1ya,this H3NO3S is Sulfamic acid.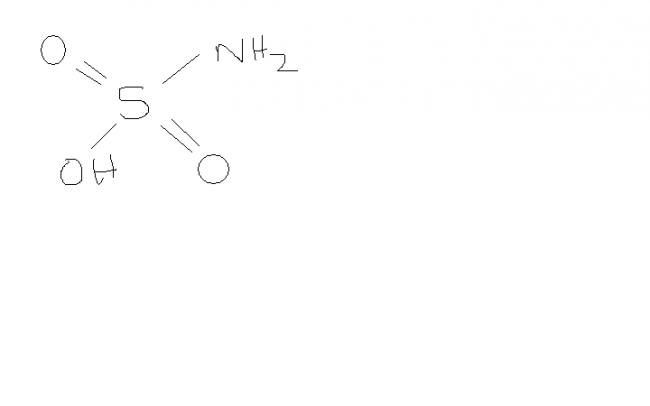
 1
1The action of conc H2SO4 on urea results in
the production of a white crystalline solid X of formula H3 NO3S,a monobasic acid. on treatment with sod nitrite and dil.HCl at 0 degree.it liberates one mol of N2 per mol of X, and on addition of aq. barium chloride, the resulting sol yields one mol of BaSO4 per mol of X taken. Deduce the structure of X.
 3
3eureka if ncert and jd lee were rite i can say dat sodium ion has lesser hydration enthalpy inorder to overcome its lattice energy.
 3
3wikipedia will made us mad.
 24
24@ msp and everyone
here is the content from wiki to support my answer
""Deliquescent materials are substances (mostly salts) that have a strong affinity for moisture and will absorb relatively large amounts of water from the atmosphere if exposed to it, forming a liquid solution. Deliquescent salts include calcium chloride, magnesium chloride, zinc chloride, potassium carbonate, potassium phosphate, carnallite, ferric ammonium citrate, potassium hydroxide and sodium hydroxide. ""
if u wanna cross-check urself ,here is the link
http://en.wikipedia.org/wiki/Hygroscopy
 3
3http://en.wikipedia.org/wiki/Category:Deliquescent_substances
 3
3eure for the second b cant be as the gp1 metals wont get hydrated.
 1
1and fr d first one, b555 hs given d perfect answer
 1
1perhaps its NH4+ because:
a) it normally forms strong ionic bonds
b) its precipitates r thermally vry stable, as they r easily formed under thermodynamically controlled conditions
 1
1The ion most difficult to remove as a ppt is
(A) Ag+
(b) NH4+
(c) Fe3+
(d) Cu2+
