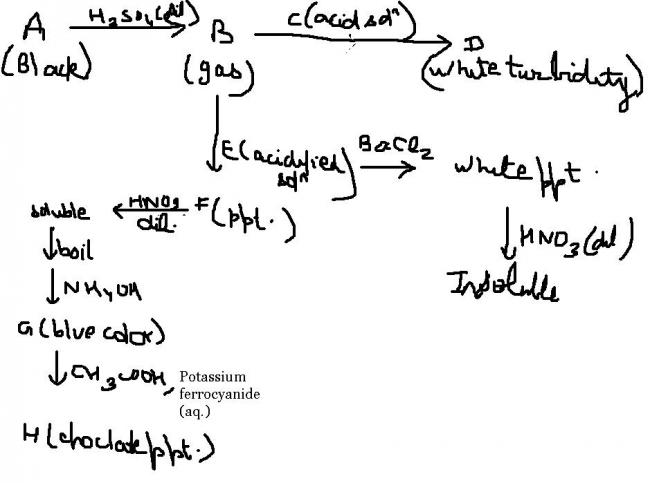
A - S2- salt , B - SO2 , C - aqueous Ba+2 salt , D - BaSO3 , E - CuCO3 , white ppt. - BaSO3 , F - CuSO3 , soluble - Cu(NO3)2 , G - Cu(OH)2 , H - Cu2[Fe(CN)6] ,
First hint frm the chocolate ppt. it needs to be Cu2[Fe(CN)6]. thus,now proceeding in the backward direction , till E , all are Cu2+ compounds.
now,its a jaggle deciding E. its sure B is SO2 and to get white ppt. frm E, E needs to hv sulphate,sulphite or carbonate. But sulphate and sulphite won't react wid SO2. Thus,its the carbonate.
but, no information about A , except that it would be a sulphide ion.