 1
1Q3 N2O + H2O
Q4 N2 +H2O
Q6 NH3
Q7 NH3 + CO2 +H2O
 24
24any reasons tush ??
and decoder i odnt have answers to those reactions.....so waitign for others to participate....but i think ur ans4 is wrong
 39
39Graphite is an allotrope of carbon, it is simply a different form of carbon where the atoms are arranged in hexagonal layers which slide on one another(giving graphite better electron mobility and thus the ability to conduct electricity). Both graphite and diamond are pure forms of carbon if not treated with any impurities.
Ans 4 is N2 + 2H2O if you want balanced eq.
 13
13When heating compounds, i first take out the max. no. of water molecules if both H & O are present in it (this works MOST of the times) [6]
4) NH4NO2 -----> (aaram se v can get 2 moles water n then only N2 is left behind) giving 2H2O + N2
3) Same way NH4NO3 --(heat)---> 2H2O + N2O
5) Glycine is an amino acid if am not wrong ; no further details in my knowledge abhi..
Also, decoder is rite fr 6 n 7 too...n i agree with pritish fr the 8th one's explanation..
 1
1Q.1) NH3 + HOCl → N2 + HCl + H2O
Q.2) The sodium ions are exchanged by calcium ions. thus, the sodium zeolite is converted into calcium zeolite. again this odium zeolite can be obtained by adding conc. NaCl solution to calcium zeolite.
Q.3) N2O + H2O
Q.4) N2 +H2O
Q.5) glycine is the simplest amino acid NH2 - CH2 - COOH.
So, when its heated it might form cyclic product.
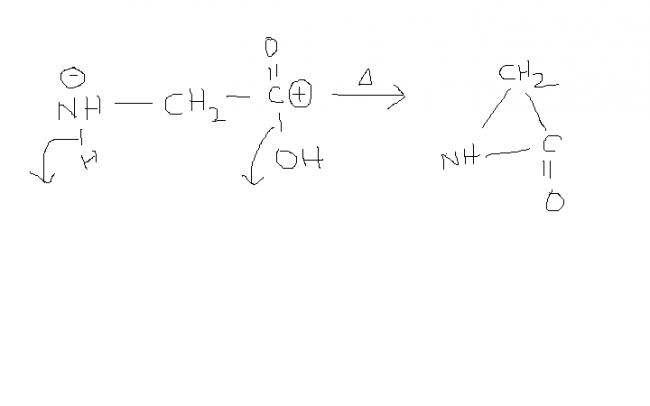
Q.6) NH3 + HCl
Q.7) NH3 + CO2 + H3O
Q.8) False. agree wid pritish ' s reason.
 24
24@aieeee
ans1 wrong
ans2 a bit incomplete
rest all correct...
and thx avik,decoder,pritish,tushar
but still Q1,2 remain unsolved
 1
1ya, ans 1 ws a self attempt. i hvn't read it till date.
Q.2) What else required ?
 24
24should i give options for Q2 ?
 1
1hehe. then its b) and d). i gave Ca2+ as the transfer is easier in it as Ca2+ zeolite is more easily transformed into sodium zeolite.
still sorry, i missed it out.
