i think the products other than the halide where gases when using socl2 so using socl2 helps to separate the alkyl chloride easily.
use of socl2 is a better approach 2 convert an alcohol 2 alkly chloride with compared 2 use of pcl5 --- please justify !!!
-
UP 0 DOWN 0 0 12

12 Answers
This is because on reaction SO2 escapes as a gaseous byproduct. Gas evolution increases entropy of reaction and from equation
ΔG = ΔH - TΔS, large change in entropy makes ΔG more negative. This means that spontaneity/feasibility of reaction increases greatly.
pritish is true -- thnx man :):) also i think hcl will also be liberated as a gas in the product side !!
@Pritish: He means HCl gets out in vapour phase. So, it makes the reaction even more feasible.
Adding to this thread: Even SO2Cl2 is preferred over PCl5 but the yield is considerably less than that in SOCl2. Furthermore, SO2Cl2 is difficult to prepare under normal conditions. That's why SOCl2 is prefrred.
Ankur the so called gaseous HCl is used up in reaction with phosphorus oxy chloride...
@Pritish... u replied that with such a confidence.. that i too got confused for a while and had to look up at the reaction in the Ncert..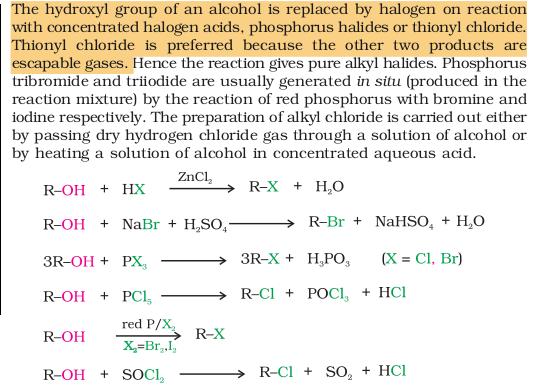
Arey I meant in reaction of PCl5 the gaseous HCl produced is used in reaction with phosphorus oxychloride...I mistook it to be PCl3, where HCl is completely used up. 1 mol HCl remains in PCl5 case.
You're right about both HCl and SO2 escaping in case of SOCl2...which one was Ankur talking about?? I don't know.
Entropy change is most in SOCl2 reaction... as there are two gases. Even then the explanation is correct?