@govind..i dont know ur source of info...but my source is http://www.chemblink.com/products/7772-98-7.htm
which is very much authentic
in question 3 both the structures are wrong..in the following image replace H by Na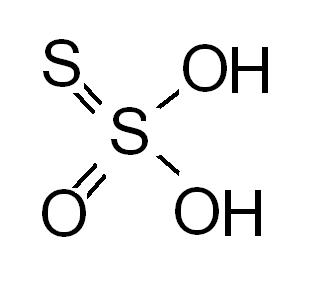
source : wikipedia
@govind..i dont know ur source of info...but my source is http://www.chemblink.com/products/7772-98-7.htm
which is very much authentic
Arrey, tht double bond is variable can be with either of the 2. (kyunki O & S ek se hi hain -groupmates)....n it aint affecting the Oxid.n state of Sulphur too, so both are valid, rather equivalent.
As for the Qn, a -ve charge will be more stable on the zero oxidation state waala sulphur - due to resonance- this resonance only makes 2 structures possible fr the formula(the two over which there was confusion).......!!
3)
In the first structure given, Sulphur and Oxygen with 2 lone pairs each are next to each other, this destabilises the molecule
The second structure by both Eureka123 and Govind are right, as all the four atoms around the central Sulphur have affinity for the Na+ions
So, they resonate.......

Source: http://en.wikipedia.org/wiki/Sodium_thiosulfate