The only primary alcohol that gives the haloform test is ethanol and the only aldehyde which gives the test is acetaldehyde.
Being a tertiary alcohol, tert-butanol(2-methylpropan-2-ol) is resistant to oxidation of -OH to =O, is my guess. Wait for more replies.
i have a doubt regarding why does 2-methyl propanol not give the iodoform test inspite of it being a methyl alcohol and how to recognise which methyl alcohol/ oxygen containing compound will not give iodoform test????
-
UP 0 DOWN 0 0 6

6 Answers
For any compound to give positive iodoform test, you need this group to be present ( or similar group which can be converted to it by oxidation)-
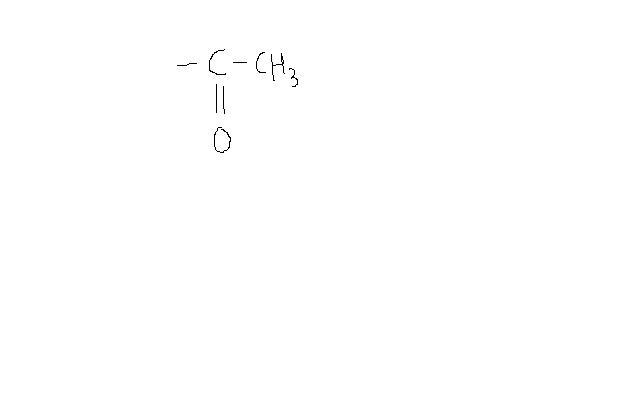 [/img]
[/img]
2-methyl propanol does not satisfy this condition. Hence it does not give positive iodoform test. Moreover, the only primary alcohol which gives + IF test is ethanol. All secondary alcohols give + test as they can be converted to compound which I showed ( by mild oxidation )
oh!! now i realise i have typed the compound wrongly and that's why swordfish i might have confused u , sorry , but i have mentioned in my heading that i am talking about 2-methylpropan-2-ol 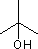 , which definitley containd CH3- C-OH type group and it ain't a primary alcohol , warna kyun puchta????
, which definitley containd CH3- C-OH type group and it ain't a primary alcohol , warna kyun puchta????
You wrote 2-methyl propanol and not 2-methyl propan-2-ol.
Anyway, this compound is a tertiary alcohol which does not give positive haloform test.
Think about it this way - can you oxidise it to get a ketone which has the group I showed? Offcourse not.
so generalising it can we say that acetone which also has has two methyl groups is non oxidisable and cannot respond to iodoform because oxidising it would mean we gotta break c-c bond??? but this is wrong!!! ( and i think after haloform we get sodium salt of acid +haloform rather than ketone!!!! )
You are not understanding what I meant.
I never told if a compound has 2 methyl groups, it cannot respond to iodoform test.
Acetone contains the group I showed, hence it gives +I.F. test.
Did I write any where that we get a ketone after haloform? How did you take it for granted?
For a compound to show +I.F., that group should be present. If you take secondary alcohol, it is first oxidised into a ketone by OI- and then there is an attack by a base.
Refer to a good book for clear understanding.