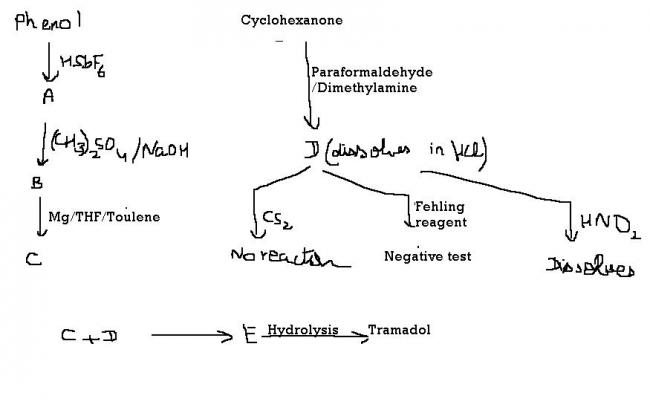
well this was in fitjee full test....and ya it looks above jee level...but can we try ??
5 Answers
Eureka...this is a little too advanced for JEE. Especially considering the usage of superacids, Mannich reactions, and the lot. These are used in complex biosyntheses..which I believe are taught in college?
Eure, you are missing a halogenation in A-B-C. Grignard reagent reacts with the Mannich base to form the Tramadol salt...that grignard reagent won't form without a halogen available.
Actually, in A, it should be X2 in presence of HSbF6. We'll get a meta halo phenol in that case. This is because phenol gets protonated by the superacid HSbF6, and OH2+ acts as deactivating group. Hydrolysis of this after the reaction yields m-halo phenol, which is A.
Now reaction with DMS gives m-halo anisole as DMS is a methylating agent for oxygen compounds. This is B.
Grignard reaction takes place for C, so it is m-magensium bromo anisole.
I think the rest is crystal clear? :) A keto group in the Mannich base(D) and a grignard reagent(C)...something clicked? :D
And ya, D dissolves in HNO2 because it forms a water soluble nitrite salt.