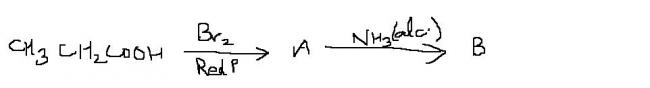
4 Answers
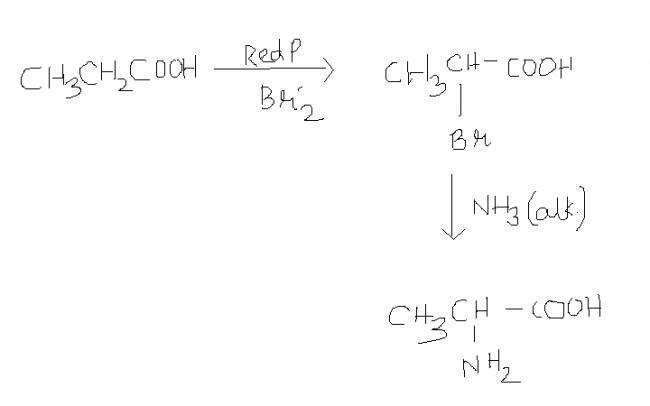
The above post solved by "aieeee" is an example of HVZ (Hell-Volhard-Zelinsky) reaction.
α-Hydrogen is replaced by halogen atom when carboxylic acid is treated with Red Phosporus and Cl2 or Br2.
R-CH2COOH + (Red P, Br2) → R-CHBr-COOH + Red P, Br2 → R-CBr2-COOH
Similar reaction takes place when Cl2 is used in place of Br2.
@ "aieeee" - Only mono-substitution takes place (even in large excess of NH3)
The product is a α-amino acid (alanine) which is very stable. Reason?
(Hint: its very simple, think which force makes the amino acids stable).
ya, right. dat ws a typo , in hurry.
i wanted 2 say in excess of Red P / Br2 , dibromo product cn be formed but even in excess of NH3
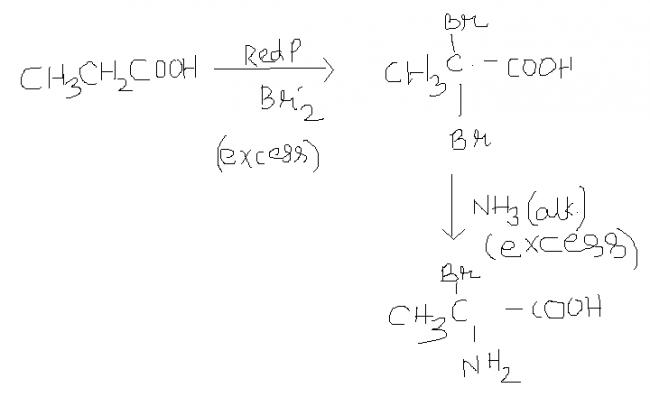
thx everyone....
actually answer given was alanine....
didnt understand what it meant [3][3]