john go thru this . hope it helps.


32 Answers
ok.......plz tell y aromaticity leads to stability of compound.......how can u say so???
yaar aromaticity leads to stability of the compound....if due to removal of hydrogen....the structure gets stabilized then that hydrogen will be easily removed....which leads to acidic hydrogen....
kis hydrogen conjugation ki vajah se structure aromatic hoga .....NOW GOT WAT IT MEANS BUT WAT HAS THE AROMATIC COMP. TO DO WITH ACIDIC H
conjugated hydrogen means hydrogen which can result into resonance structures by conjugation.....
hydrogen conjugation means resonance structure formed due to transfer of electron of C-H bond....
PLZ TELL WAT IS MEANT BY HYDROGEN CONJUGATION AND CONJUGATED HYDROGENS
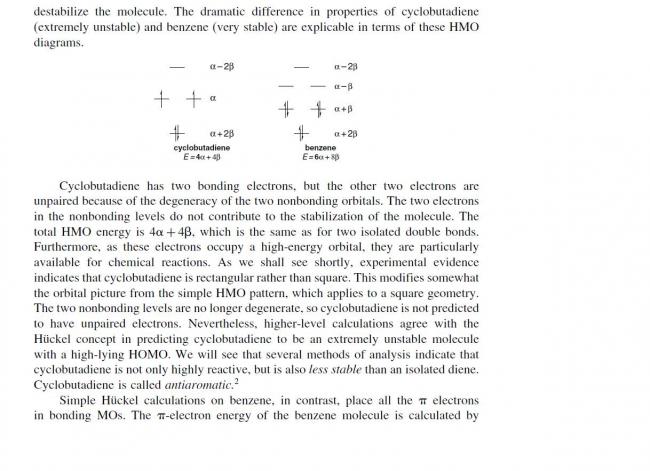
aromatic means..
a) delocalised π bonds...
b) (4n+2)Ï€ electrons...
c) every carbon must be sp2 hybridised.....
these are conditions for aromatic character.....
kis hydrogen conjugation ki vajah se structure aromatic hoga
even celes told that.but i din get wat it means
yaar dekh sabse pehle toh yeh check kar le kis hydrogen conjugation ki vajah se structure aromatic hoga...agar nahin toh phir jiska resonance stucture jyada stable hoga that hydrogen will be more acidic....
ok.......so this was sort of eliminating options which we did in #14.
can u suggest how to approach so that we directly come to know which r acidic hydrogen.
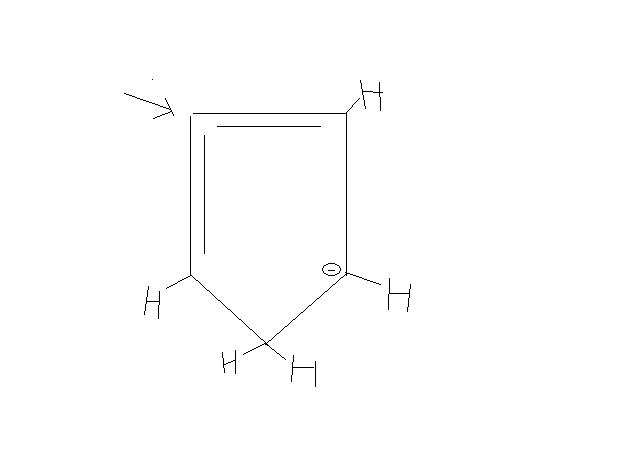
THE CARBON REPRESENTED BY ARROW SIGN IS "sp" HYBRIDISED...so it is the bond angle is 180° which means linear structure.....therefore the structure doesn't remain cyclic....
HOPE U'll UNDERSTAND BY THIS....IF ANY DOUBTS PLEASE ASK.....
THE IMAGE IS THE CONJUGATED STRUCTURE FOR HYDROGEN PRESENT AT CARBON REPRESENTED BY ARROW SIGN....
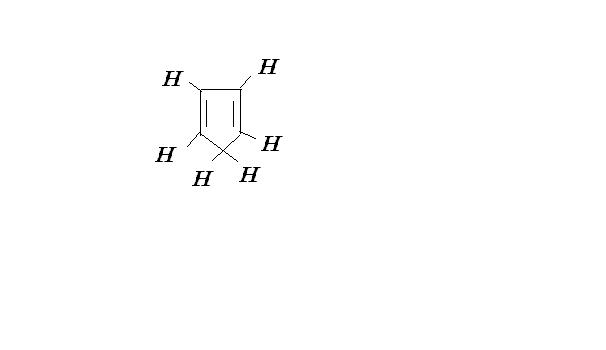
think about the conjugation from those hydrogen which u have encircled......
try drawing the resonance structure of it.........
there will be two double bonds around carbon containing that hydrogen....
thus it will be sp hybridised.....which means 180° thus the structure won't remain cyclic.....
WAIT FOR MINUTE I'll TRY POST THE IMAGE.....
u r right prateek.but din get ur explanation.
plz xplain in a bit detail
conjugated ones are stabilised by resonance hence the carbocations are stable...hence acidic..
i thnk the ones which u have left becoz....
if the ones u have encircled is taken into conjugation then in the first resonance structure the carbon will be sp hybridised....i.e. 180°
therefore structure will not be cyclic....
therefore according to me it shud be the ones which u have left....
Also due to the conjugation the structure acquires aromaticity....
no the ones which arent circled
moreover if u rmove H+ from there u get a aromatic comp