i m sure it is dibasic ,
the pKa for first proton is 1.5 and for the second one is 3.4 , and both protons are more acidic than acetic acid ka proton , since the anion formd by removing 2 H is more stabilized than the anion formed by removing 1 H
Consider the following compound A (below) : -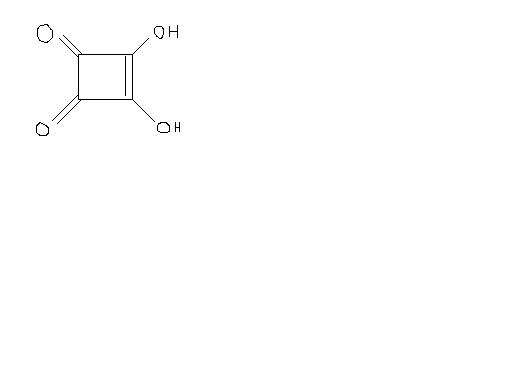
Select the correct statement(s) :
(a) It is more acidic than CH3 OH
(b) It is more acidic than CH3 COOH
(c) It reacts fastest with Lucas reagent
(d)It is a dibasic acid
-
UP 0 DOWN 0 0 17

17 Answers
Some more info
diff names ::
3,4-Dihydroxy-3-cyclobutene-1,2-dione;
3,4-dihydroxy-cyclobut-3-ene-1,2-dione;
3-cyclobutene-1,2-dione
Squaric acid is an unusually strong acid for an organic acid.
It's also unique because of its strained ring. In general, five- and six-membered rings dominate in chemistry - hence the endless parade of hexagons. Higher rings are tolerated but not-so-favored. Lower rings are possible, but, again, unfavored, because one tends to find atoms' electrons in clouds that don't overlap so well in this configuration. Along with some other moderately strong organic acids (such as the ubiquitous "alpha-hydroxy acids"), it's attracted some attention for (prescription) dermatological use.
Tushar i think thats bcz the institute copied the question and ans both frm arihant lol
So this is another exceptional case where tautomerization is quashed in favour of resonance.
lol I googled squaric acid and below the Wiki link I got -:
#
Squaric Acid For Wart Removal
#
Squaric Acid Treatment for Warts, Health Facts For You, UW Health
#
alopecia areata - topical sensitizers and contact sensitization ...
#
Squaric Acid Wart Therapy
So much for checking dibasicity.
I found one confirming link however -:
Syntheses and Derivatives of Squaric Acid
The acid is insoluble in the usual organic solvents. Squaric acid is dibasic, pK2 = 2.2, pK1 = 1. In the resonance- stabilized dianion (2), all four oxygen ...
doi.wiley.com/10.1002/anie.196608881 - Similar
@Qwerty and Ishan
But I checked the answers of both DIFFERENT coaching institutes study materials, and they had given the ans to be (a) , (b). In ARIHANT ALSO, ans is given to be (a) , (b).
it is dibasic the reason being plain and simple: both the OH groups can donate H+ as they will be resonance stabilized
and i agree with qwerty the answer is a,b,d
and wats d resaon for it ?? mention that too along with it , as i did wen i mentioned it to be dibasic [6]
One of the -OH groups will become a keto group. So it isn't a dibasic acid, it should be a monobasic acid.
It being a secondary alcohol will react with Lucas reagent in five minutes. Faster than what? What are you comparing with it?
Dunno about above two...
Well, I found that the above compound A (also known as squaric acid) is a good acid bcoz after losing H + , it gets high intermediate stability.
http://en.wikipedia.org/wiki/Squaric_acid
okk.. draw the conjugate base..
it is completely delocalised with high number of equivalent resonance strucutres.. so it is more acidic than methanol as well as acetic acid
I also think it should be a dibasic acid.. as there is even more resonance.
Anhydrous zinc chloride in HCl is Lucas reagent. It reacts fastest with tertiary alcohols.
it shud be a,b,d, i dont kno wat is lucas reagent, so cant coment abt c option
It shud be a dibasic acid , since the anion formed by removing 2 H atoms will be highly stabilised due to resonance
I'm a bit weak in 11th class concepts, and that includes tautomerism. Could you elaborate, Asish? I didn't completely get you..
but pritish, wont converting into a keto form become less stable as the enol form is stabilised by a 5 membered ring like... Hydrogen bond?
That's why I was thinking that conjugate acid wont be stabilised as one of the H-bond breaks.. (altjhough inductive effect favours acidity)