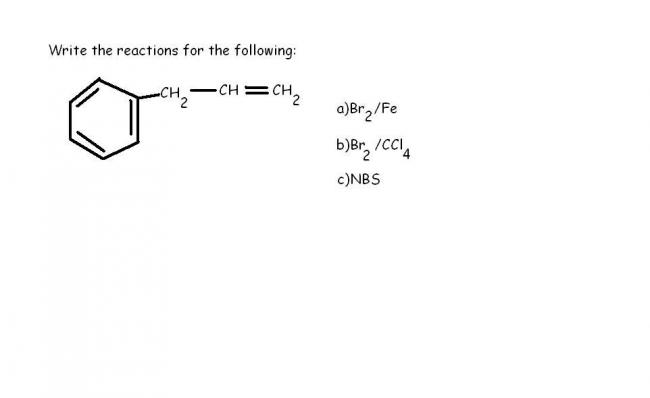
hey why para product??
5 Answers
Akshay Pamnani
·2009-01-16 23:19:17
a)Substitution of Br at meta position
b)addition of Br at carbon containing less hydrogens(more stable intermediate)
c)substitution of Br at allylic or Benzylic position both same in this case
voldy
·2009-01-17 00:23:57
a) predominant para product.
b) Br2 adds to the double bond of alkene outside benzene ring.
c)bromination of α-position wrt the double bond.
this should suffice. I think.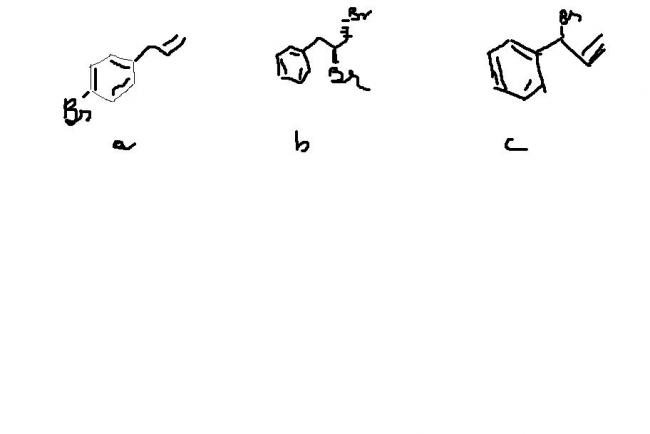
prateek punj
·2009-01-17 01:16:25
a) the first one should be the product containing br at meta position....
b)the second one is attachment of br to -CH- of the double bonded straight chain....
c)the third one is the attachment of br to benzylic carbon....