Could you upload the image to a site other than imageshack??
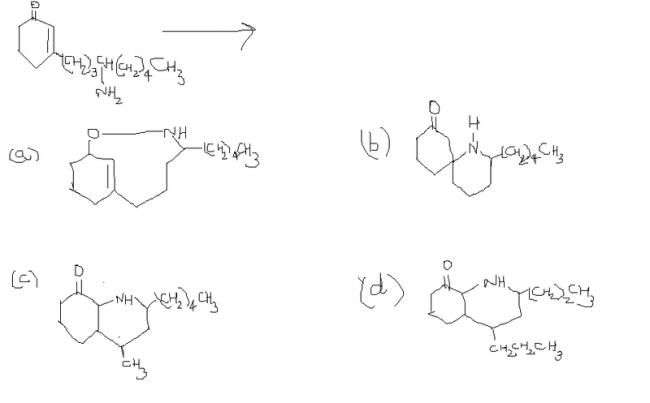
Following compound A spontaneously changes into
http://img846.imageshack.us/i/51646289.png/
ONE OR MORE THAN ONE OPTIONS MAY BE CORRECT.
-
UP 0 DOWN 0 0 8

8 Answers
Could you upload the image to a site other than imageshack??
ROFL hahahaha
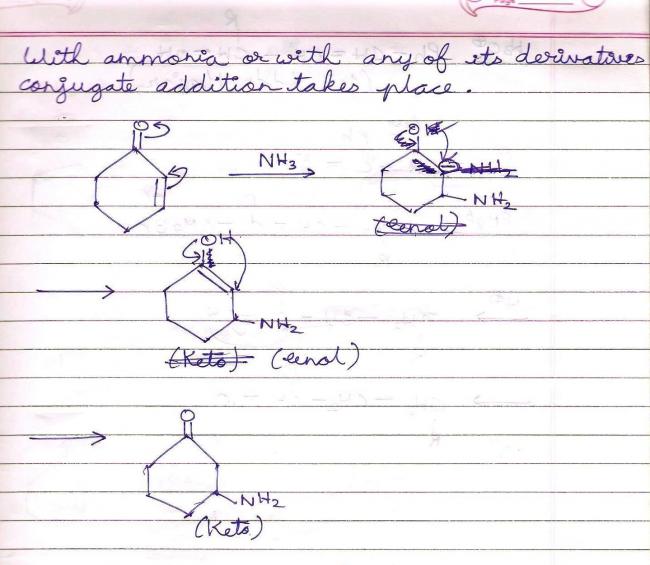
Spontaneous transformation means that there's obviously a stabilizing (read : six membered) transition state and some quick tautomerization involved. I'd put my money on b) being the answer in that case.
After the tautomerisation has taken place according to Pritish Bhaiyya ' s mechanism , obviously there will be a development of " negative charge " on the " α " carbon and a development of " positive charge " on the " β " carbon . Since " - N H 2 " is an electron releasing group , hence it ' ll try to stick to the " β " carbon only , because if it doesn't , then the density of " negative charge " on the " electropositive carbon atom " would increase . Hence , I think " ( b ) " is the correct option .