kyun ?
Assertion : A is more stable than B .
reason : Resonance effect leads to stabilization .
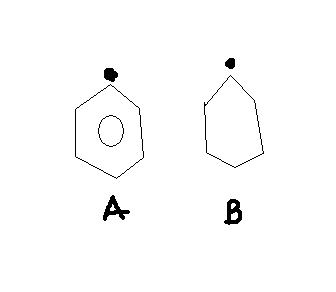
-
UP 0 DOWN 0 0 4

4 Answers
skygirl
·2009-03-25 19:27:33
yes it is D.
the carbons are all sp2.. so more electronegative..
so any positive charge or radical on them is not stabilised by them.
but in II, the radical is stabilised by the sp3 carbon atoms.
+
resonance is possible only for conjugated system..
any positive / negative charge 'on' the banzene ring will not take part in resonance.