1>2, and 3>4
but not sure how 1 and 3 compare
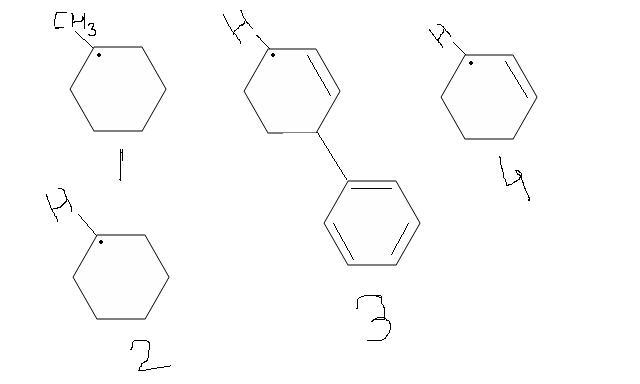
Arrange them in increasing order of stability With reasons.........
-
UP 0 DOWN 0 0 16

16 Answers
1) 3° free radical
2) 2° free radical
3) allyl free radical with more resonanating structures
4) allyl free radical with less resonating structures.
3>4>1>2
allyl radical > 3° > 2° > 1°
more resonating structure > less resonating structures.
I don't see how the 3rd radical is any different from the 4th. The radical doesn't delocalise over benzene, try making the structures and see for yourself! Had benzene been attached to the double bonded carbon, the 3rd radical would be the clear winner in this little competition.
I would rather say 3 = 4 > 1 > 2.
EDIT::
1) 3° free radical
2) 2° free radical
3) allyl free radical
4) allyl free radical.
3=4>1>2
allyl radical > 3° > 2° > 1°
@pritish, doesnt phenyl grp have a weak -I effect?
so, maybe it would be 4>~3>1>2
well the answer should be 4≈3>1>2 :
REASONS:
1.inductive effect dies out after three bonds!
2.inudctive effect due to phenyl grp is weak -I effect!
REASON FOR 1>2:
1.we see tht 1 has a -CH3 group too which adds on to the stability of 1...so 1>2
Possibly so asish, but Ph's -I effect is very weak. I would still go with 3 = 4. Euracle that answer is not possible. Had Ph been attached to the double bonded carbon instead, it would've been the answer you mentioned.
wat are u guys talking here about ?
why are u guys even considering -I effect on a species wich is neutral ?
i think i will go with 4 > 3 > 1 > 2
bcz HC structures in 4 > 3 ....
in no 3
for ph group no of resonance structures is 3+1 =4
so its more stabilised
therefore i would go with
3>4>2>1
kalyan can u please show me how is the radical resonating with the ring ?