Cl ke jagah par NH2..
13 Answers
tapanmast Vora
·2009-02-19 08:17:18
MAYB NOT
ACTIVATOR WINS OVER DEACTIVATOR SO SHUD BE ORTHO TO CH3 but not b/w NO2 n CH3, on the other side...
ans kya hai?
Sunil Kumar
·2009-02-19 08:45:13
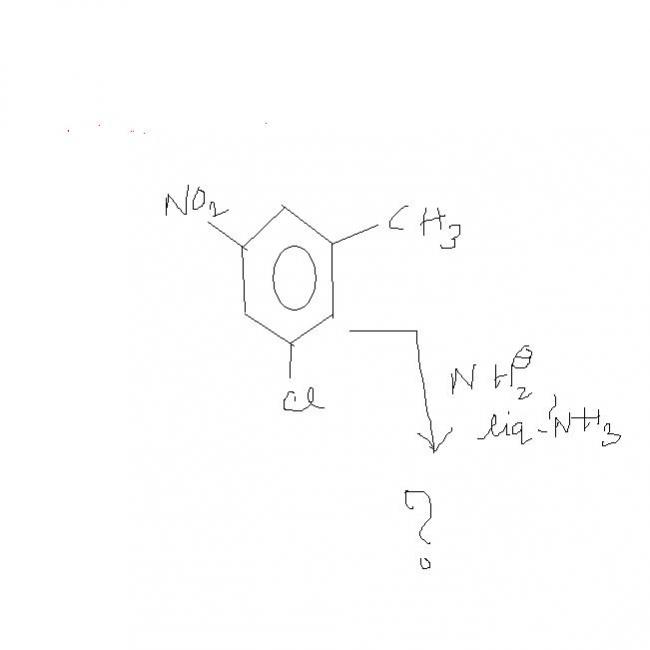
See the intermediate formed can have 2 structures:-
and correspondingly following products can be obtained
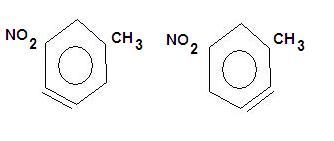
Also there is carbanion formation in between so that structure will be favoured which is obtained from most stable carbanion...
Sunil Kumar
·2009-02-19 09:01:18
In my view the NH2 will be at place of Cl cause NO2 group is -I group hence it can stabilise the carbanion formed but on the other hand it is also a deactivating group.
vector
·2009-02-19 09:03:11
i just thought dat as m effect dominate over H n I Effect so it ll be favoured to get rid o f -m of no2