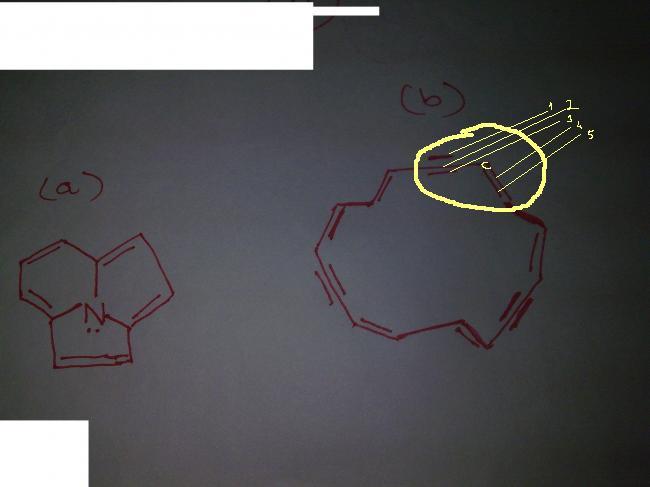
how is the circled portion possible..carbon is group 2.....how can it form 5 bonds???
WHICH OUT OF A AND B ARE AROMATIC???????
ANS : BOTH ...... PLZZZ XPLAIN Y??????
CHALO BAS EDIT BHI KAR DIYA.....
NOW IS IT FINE!!!!!!!!!!!

how is the circled portion possible..carbon is group 2.....how can it form 5 bonds???
ya...first one is surely aromatic...
but i think the second structure is not possible altogether...such a huge ring cannot be planar....
ok...didnt see the editing......now seems to b correcr........n the first structure seems aromatic too.......so first is aromatic....i was confused abt the nitrogen part since nitrogen lone pair isnt takikin part in d conjugation.......
@tapanmast
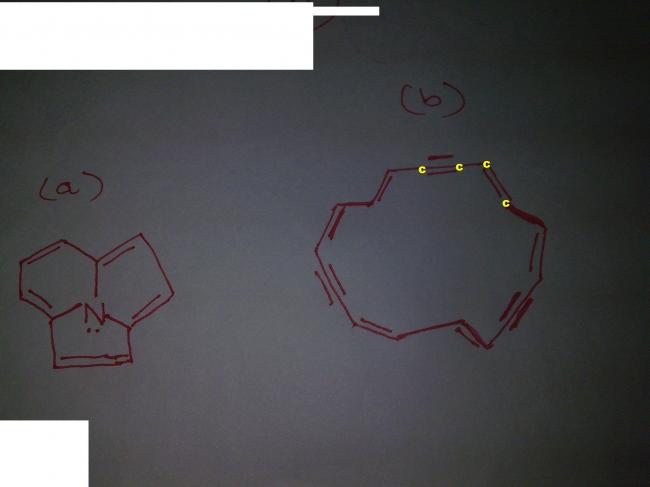
can u reedit structure 2 and name the carbon
so that it is much more clear
the second structure does not follow the 4n+2 pi electron system rule so obviously not aromatic.
@ROHIT
THE ENCIRCLED CARBON IS DOUBLY BONDED TO THE CARBON ON LEFT AND SINGLY BONDED TO CARBON ON THE LEFT(WHICH IN TURN IS TRIPLY BONDED!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!)
ROHIT HOPE U HAV CN THE CORRECT ANSWER;
THE ANSWER IS DAT BOTH ARE IN CONJUGATN!!!!!!!!!!
CAN U XPLAIN Y WER U SUSPECTIN STRUCT 2 TO B NON AROMATIC???????
hehe..thats y i said..its non aromatic......not possible only.......so.......
n abt the first one....isnt conjugation missing in the lowest ring???
GUYZ PLZ DONT MISREAD THE QUESTION.....
STRUCTURE IS LIKE THIS :  AND THEN THER IS A CARBON WHICH IS DOUBLY BONDED, I.E. THE ONE YOU HAV ENCIRCLED!!!!!!!!!!
AND THEN THER IS A CARBON WHICH IS DOUBLY BONDED, I.E. THE ONE YOU HAV ENCIRCLED!!!!!!!!!!
HOPE U GET THE STRUCTURE NOW!!!
For aromaticity
1)there should be 4n+2 pi electrons.
2)Structure should be planar
3) pi electrons must be in conjugation
In structure 1
10 pi electrons ≡ 4 X 2 + 2 electrons
4n+2 rule satisfied with n=2
All the electrons are in conjugation
structure in planar
So Structure 1 is aromatic
and b/w if you include the lp , then won't it become antiaromatic ?????[7]
yes u guys r rite, i forgot dat the lps are included in resonance.and tx for correcting me
sankara,
while chking 4 planarity u jus take into account da bond pairs.......
take eg of pyridine [AROMATIC] it too has LP of electrns perp to plane of da ring yet its aromatic.....
so that cant b a reason for a) being non aromatic.......
as it is da ans says its aromatic.......
though i am weak in this part........but sankara...LP enters resonance so it becomes sp2 hybridised ..
i think none of these shud be the answer;
because for the option
a)it doesnt have a planar structures because the lone pair of electrons on the nitrogen atom is perpendicular to the plane of the paper
b)the structure doesnt have an uniform electron density throughout the molecule.
please correct me i know my ans are wrong....
but this is the ans wat i got......
thats what i thought..becoz then b clearly wouldnt have been aromatic.....
ther is no wedge bond..... its a planar struct
jus darknd it by mistake....... lol......