arey no sorries reqd,,,,,,,,,i am not a rude character....[3]
44 Answers
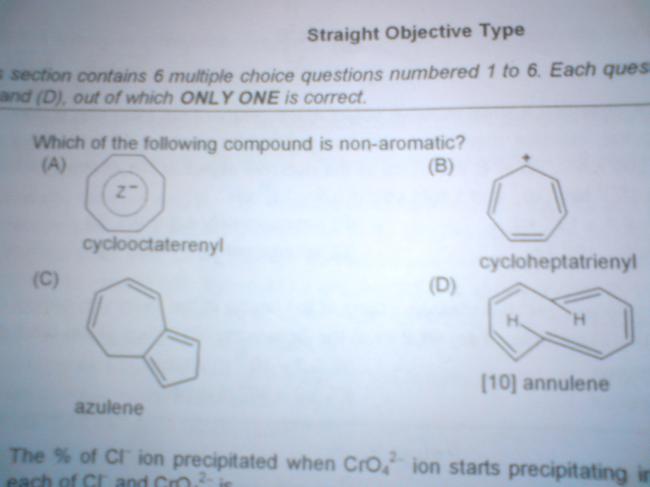
i searched up the real structure of azulene ,and found this

which means fiitjee messed up with the structure of azulene,sorry guys for the confusion
post # 27.......................uff kehne se pahle ckeck to kar lo sky /star/girl......whatever........[3][3]
arey dun take dat seriously ....
am sorry if i had hurt ur senti ...
pls forgive... [17]
yea.. D is non-aromatic.. annulene-10 is not aromatic.. exception 2 huckle's rule..
i have read dis.. this is because the trans -annular hydrogens(d 1s shown separately in d figure) stearically interfere wid each oder n push d molecule out of planar..
since its not planar, resonance of pi electrons(evn tho its 4n+2) is nt possible..
half of d molecule is above n half of it is below d plane..
this is called stearic inhibition of resonance..
** annulene-14 is aromatic despite not satisfying huckle's rule..
good.. about annulene 10...:)
but annulene 14 me 14 pi electrons hote hai naa..it follows huckel rule for n=3 (4.3+2)
but annulene[ 10 ] has two more structures and none has that trans-annular Hydrogen but still they are non planar.. due to angle strain... :)
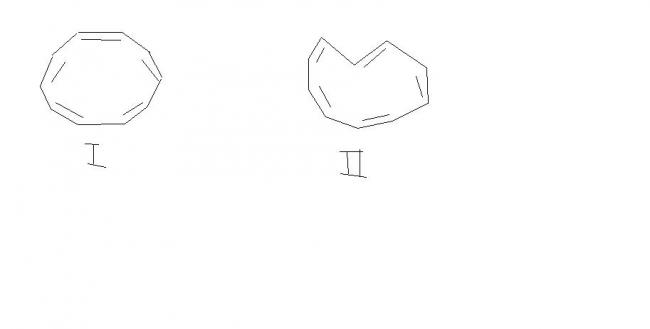
structure I has all cis double bonds.. but as angle in planar structure would be..1440 so due to angle strain its not planar...
similarly if one is trans double bond...(II) then also there would be angle strain...
and one having 2 trans double bond is previously shown (having trans-annular Hydrogen..)
:)
yea.. thnx 4 dat update.. bt acc to wat i've read annulene-14 has 16 pi electrons.. u can check it up to confirm tho.. do lemme knoe if there's sm update ..
c par kis baat ka bawal hai usme to conjugation hi nai hai so c bhi non aromatic hona chaiye
although structure given is wrong bt we hav to ans acc to the given data i suppose??
THE ANSWER IS B! SINCE DUE TO AROMATICITY THE MOLECULE ATTAINS EXTRA STABILITY THEREFORE TO GAIN THE STABILITY THIS MOLECULE COME IN THE PLANE AND HENCE SATISFY ALL THE CONDITIONS OF AROMATIC!!!!!!!!!
UNDOUBTEDLY THE ANSWER IS B!!!!!!!!!
KYON BHAI VIVEK SAHI HUN NAAA?????????
10 ANNULENE SUFFERS STEARIC HINDERANCE DUE TO THE HYDROGEN ATOMS AND HENCE BECOMES NON PLANAR AND HENCE IS NON AROMATIC!!!!!!!!!
A is a COT structure and hence this is non planar and hence is non aromatic!
this is having a boat like structure!!!!
C is non conjugated hence that cannot be the aromatic species that is why i said undoubtedly B is the answer!!!!!!!!!!
cheers!!!!!!!
kaise C aromatic hai ????
no of pi-electrons = 8 !! i.e.4n ...
pakka anti-aromatic.