2>4>3>1 ??
arrange the following in decresing order of stability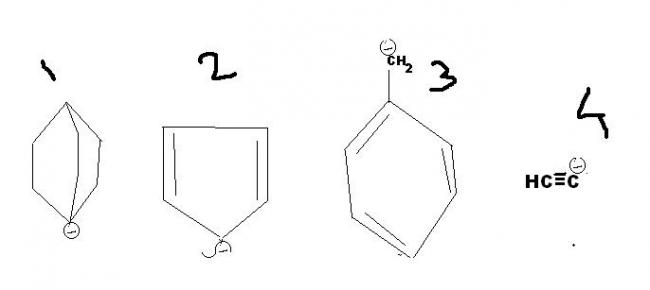
-
UP 0 DOWN 0 1 10

10 Answers
2>3>4>1
2 is involved in resonance
3 is benzylic So quite stable (resonance dominates sp hybrid)
4 is sp hybridised. so the negative charge is more stabilised
1 is sp3 (least EWG)
bcoz in 2 the resonating str is i one plane but in 3 ch2 is in other plane
please explain how in 2 and 3 different planes can affect stability.
i think in 3 there will be more delocalisation.....so itshould be 3>2........
ashish can u plz explain
Arrey bhai, "2" is Aromatic.....Cyclopenta dienyl anion - Hence 'tis the Stablest...
Baaki order i agree ,wud be- 2>3>4>1.
2>4>>3>1 as bridge carbanion is least stable 1 are bis leastt stable and as 2&3 are resonance stabilized but in3 the carbanion is out of plane.so 2>3