Can this be explained in terms of I effect?
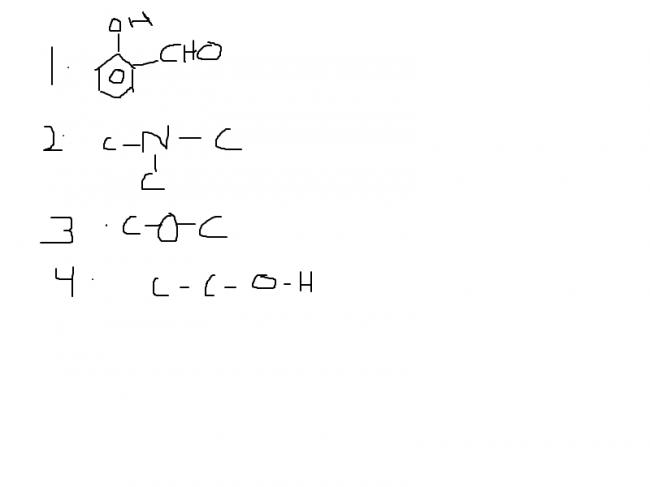
The given arrangement has to be arranged in increasing order of water solubility.
Why is the answer 4>3>2>1?
In 1, chillation occurs.
and i have some idea about 4 being a the top.But still, can someone please clear my doubts? In particular about the 2nd option.
-
UP 0 DOWN 0 2 2

2 Answers
as 4 th one can form H bonding with water molecule in greater extent than others hence solubility is most in case of it
then as for 2nd and 3rd ase as ether can form stronger H bonding thn 3°amine so its solubility z more
and in case of first one as benzene is electron withdrawing group and an another EW group iz attached to it so electron density on O is less hence it can form very week H bond
hence solubility order iz as given