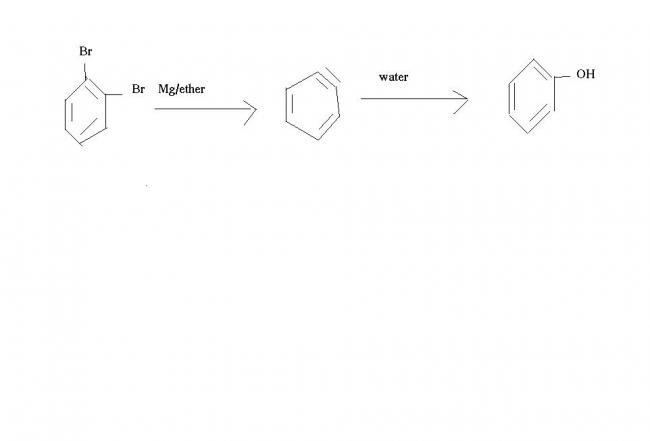
it needds angle of 120* and is having only 60*
pls tell the product...
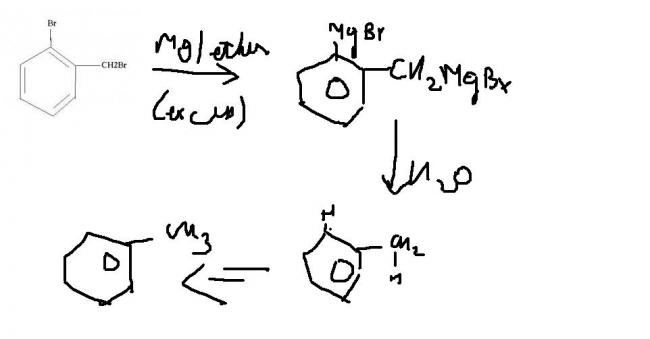
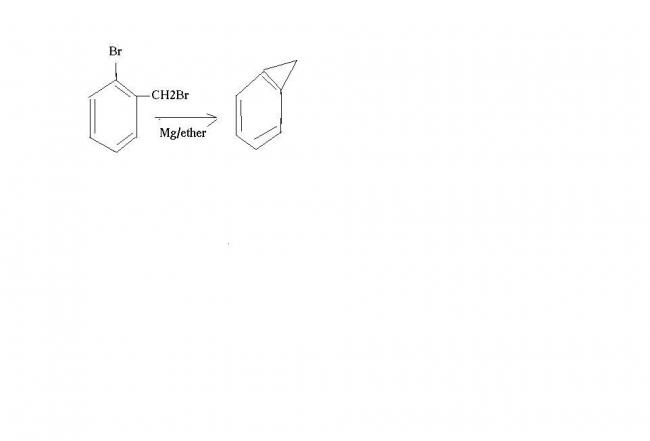
1. Mg/ ether (excess)
__________________
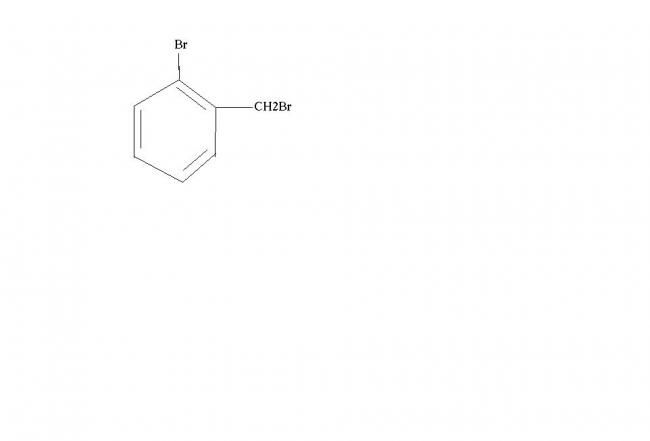
o-Bromobenzyl bromide ?
2. H2O
-
UP 0 DOWN 0 0 25

25 Answers
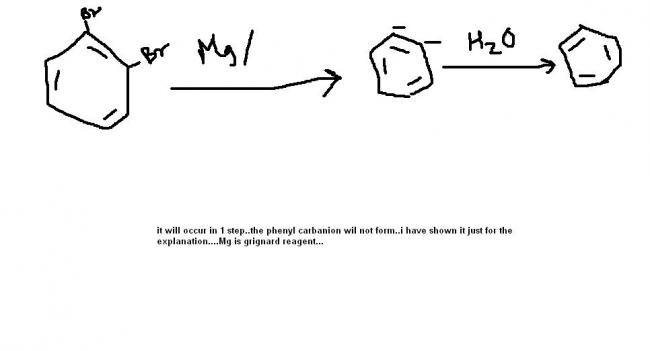
ok now think if it was not mg/ether in excess. Then which Br will be attacked will be important
No the answer will be
p-methyl Benzene
Since first it forms a grignard reagent and in G.R mgX have a positive charge
Therefore it will form first carboanium ion and then react with H+
grignard causes a - charge n then since water is there...H+ is taken...simple:0
then it is rather more easy..just remove the br and put H...i dun think there is any chance of forming a three member ring!!
that was mah post no.8 n i too gave the same product..[:P]
ok... may be.
i dont know, i thought this should be correct as it was a dihalide.
i dun think so..i though of dis onen i mentianed in mah previous post that thiswill unstable due to the strain!!though m not very much sure
u cannot have a alkyne since the strain in d benzene ring will be tooo much then!!
arre i meant that onnly that it will form grignard wid Mg...n Mg/ether is called grignard reagent!!
Mg is not grignard. Grignard is RMgX.
with the help of Mg and dry ether along with alkyl halide we form grignard reagent.
1,2 dihalide's rxns give alkenes ( here, 1,2 dihalide alkene type rxn gives alkyne ) with Mg/ether.
then it is rather more easy..just remove the br and put H...i dun think there is any chance of forming a three member ring!!
benzoine intermediates reaction doesnt occus...n here if wht u r telling is right shreya then WHY HAVE THEY GIVEN EXCESS GRIGNARD???
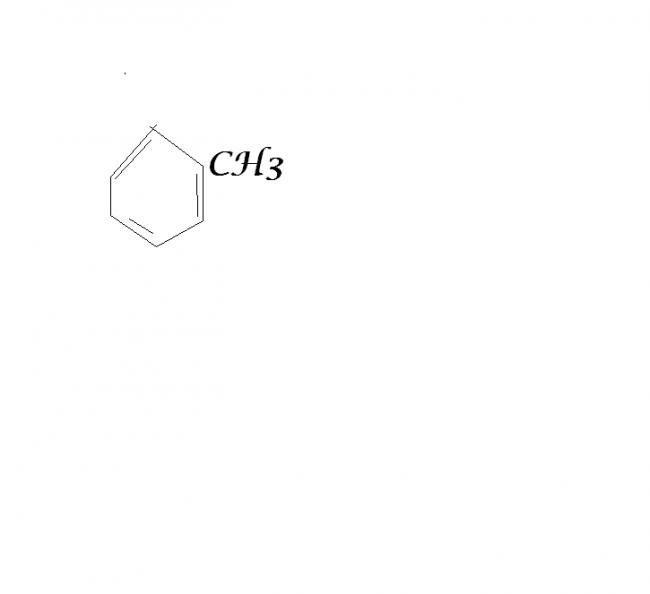 I think this Will be the correct answer
I think this Will be the correct answer