the most stable intermediate will be fourth one because it is in conjugation so charge is stablised.........n then the 2nd one because it is oalso in conjugation .....but a bit lesser then the 4rth one....after it i think it must be 1 as the other two r very unstable.........
then 3 n then 5th.......
how to understand which of a free radical is stabler??
we know that conjugated system is stabler than 3° is stabler than 2° which is stabler than 1° and ultimately weakest is .CH3
but what if the question involves things like this...
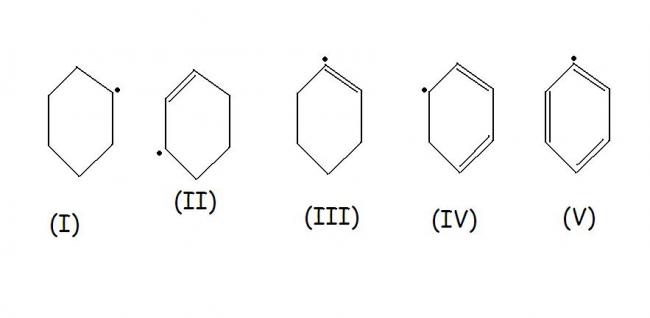
please reply with complete reason and please reply soon...its urgent...[2][2][2]
-
UP 0 DOWN 0 1 8

8 Answers
Tapas is correct...4 and 2 are allyl radicals, 4 is doubly so. Thus they are stabilised by resonance. Radicals are similar to carbocations, only their mechanisms of reaction are different.
5 is the most unstable radical because benzene's aromaticity is being hindered badly by loss of that electron. To gain an electron will be an exoergic process(energy releasing because it will be favoured highly).
Similarly 3 is also unstable because its a vinyl radical.
Thus 4>2>1>3>5.
oh my god...how the hell is (IV) conjugated??
i thought conjugated systems have alternate single and double bonds???[2][2][2]
help out!!
In 4, the radical is on the Carbon atom that is single bonded to the double bond C..alternating.