I KNOW THE REASON IS HYPERCONJUGATION BUT HOW???????
16 Answers
donation or complete shift of CH bonded (sigma) e-s to the adjacent atom is called bakernathan effect or hyperconjugation
also called no bond resonance(no bond between H and C) but it will be there due to att.force
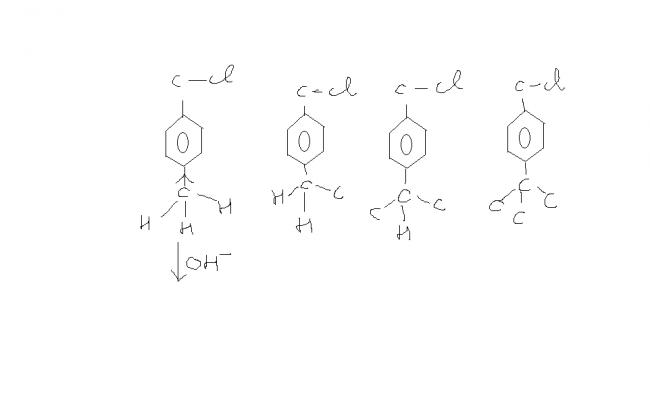
in all dese fig
1) more hyp. conj due to
3 Hs-----more decrease in +ve
charge
2) 2Hs---- decrease in +ve charge
3)1 H ----less decrease in +ve charge
4)no hydrogen so zero hyperconjugation
he thought it was due to inductive effect
but the observed order was 4>3>2>1
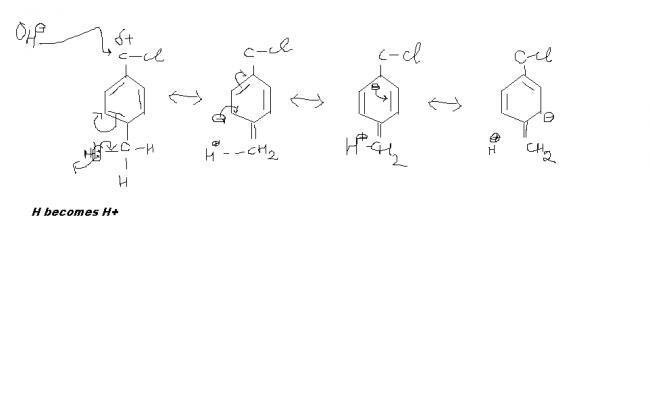
this decreases he +ve charge on the C by donating more e-s increases the e-n density
this is for 1 H ...but there are 3 H's. high hyperconjugation
therefore as there is more +ve charge on carbon ,OH will attach more easily and hence rate
shud b increasd.in case I more H.C is possible due to 3 alpha H and hence less pdt.but in IV no alpha H so zero Hc and thus the rate
of ther reaction is high
so generally
RE > HC > IE
(reso) (hyper) (inductive)
pi bond sigma bond partial shift
easy cleavage cleavage diff sigma bond
non bonded e-s complete shift
complete shift ivolves p-orbitals
@#7
aarthi are u sure that rate for IVth is highest??????i read exactly opposite!
which buk diid yu refer @john ?? refer solomon i think it also says the same
HC increases the stability of the carbocation in general......and more the stability lesser the reactivity.....and it applies to this too
hey aarti although this is a silly doubt:::::[3]
can u tell me wat is alpha carbon and alpha hydrogen!
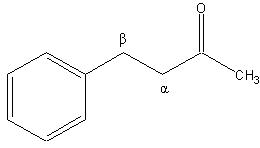
Alpha and beta carbon
From Wikipedia, the free encyclopedia
(Redirected from Alpha carbon)
Jump to: navigation, search
The alpha carbon in organic chemistry refers to the first carbon that attaches to a functional group (the carbon is attached at the first, or alpha, position).[1] By extension, the second carbon is the beta carbon,[2] and so on. This nomenclature can also be applied to the hydrogen atoms attached to the carbons. A hydrogen attached to an alpha carbon is called an "alpha-hydrogen" (α-hydrogen), a hydrogen on the beta-carbon is a beta-hydrogen, and so on.
This naming standard is sometimes considered to be not in compliance with IUPAC nomenclature (which encourages that carbons be identified by number, not by Greek letter); but it nonetheless remains very popular, particularly because it is useful in identifying the relative location of carbons to other functional groups (often a carbonyl).