i think it should be (C)
In presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti markonikov's addition because:
options are as:
-
UP 0 DOWN 0 0 8

8 Answers
With hydrogen chloride, the second half of the propagation stage is very slow. If you do a bond enthalpy sum, you will find that the following reaction is endothermic.
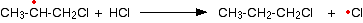
This is due to the relatively high hydrogen-chlorine bond strength.
it has been experimantally found....
wat to explain.... thats the reason....
@skygirl: yeah it has been experimentally found out yet abhirup has found the problem that i was facing! he actually wrote the step which is endothermic and hence he cleared my doubt without performing the experiment so the doubts can be cleared without performing the experiment!!!!!!!
cheers!!!!!!!!