Q3)the given IUPAC name is wrong.: 3- Methyl-4-ethylheptane.what is the correct IUPAC name?
Its correct name is 4-Ethyl-3-methylheptane.
while doing nomenclature of the substituents you should take them in alphabetical order(here e comes before m)
Q1)For detection of hydrogen and carbon in compounds why do we take Cuo three times the weight of the organic compound?
Q2)What decides how many parts of compound are to be reacted with how many parts of reagent.
Q3)the given IUPAC name is wrong.: 3- Methyl-4-ethylheptane.what is the correct IUPAC name?
Q3)the given IUPAC name is wrong.: 3- Methyl-4-ethylheptane.what is the correct IUPAC name?
Its correct name is 4-Ethyl-3-methylheptane.
while doing nomenclature of the substituents you should take them in alphabetical order(here e comes before m)
Q1)For detection of hydrogen and carbon in compounds why do we take Cuo three times the weight of the organic compound?
A small quantity of pure and dry compound is mixed with about ten times its weight of
copper oxide(CuO). The mixture is taken in a hard glass test tube fitted with a delivery tube
having a small bulb. The other end of the tube is immersed in freshly prepared lime water. In
the bulb of delivery tube, a small amount of anhydrous copper suphate is placed. The mixture
of qualitative analysis heated strongly when carbon and hydrogen present are oxidised to
carbon dioxide and water respectively.
C+2CuO -> CO2 + Cu
2H + CuO -> H2O +Cu
Hope this clears your query. [1]
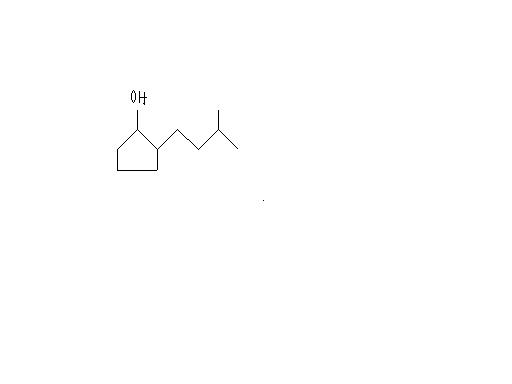
what is the IUPAC Name.
In resonance material it is given primary prefix is cyclo.isnt it wrong?Shouldnt the root word be cyclo.