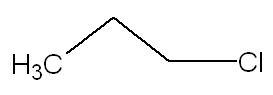
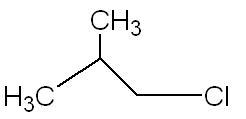
21
21For the 4th one :
we have to check the stability of the conjugate base of course. Reactive methylene compounds have highly acidic α hydrogen. An example of such a compound is 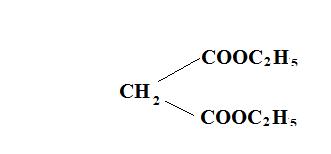
Because it's conjugate base is resonance stabilized from both sides. Knoevengel Reaction is the reaction of these compounds in presence of NH3 or its derivatives.
 49
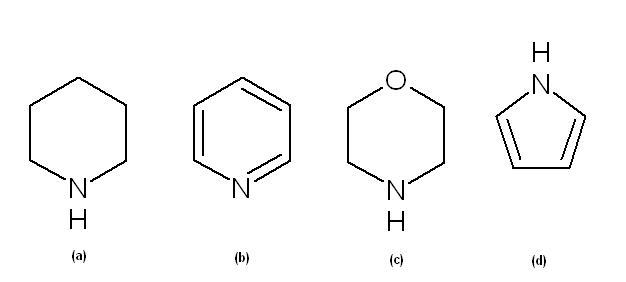
495. a>c>b>d
Why is d least?
in d N is sp2 hybridised..
two of the three sp2 orbitals is bonded to C and the third is bonded to H
thus the lone pair exist in the p orbital and is utilised in dislocation by resonance and hence is not available for donation.. thus least basic..
in b, the lone pair exixts in a sp2 orbital and in a,c it exists in sp3 orbital..
sp2 hybridised N is more electronegative than sp3 hybridised N
thus, the N in b has lower tendency to donate the electron pair than the other 2..
thus it is second least basic..
in a and c..
In c the more electronegative O atom pulls the electron cloud by inductive effect away from N creating a ∂+ charge on it, thereby reducing it's tendency to donate the electron pair.. thus it is less basic than a!
thus the order:
a>c>b>d !
cheers!
I am so happy ki abhi tak yaad hai mujhe ye sab! :D pata nahi kab tak rahega! :(
 49
493) false.
It doesnot take place becaus the electron cloud in C-H bond is at a large angular distance from the required spot (120°)
hyperconjugation is only seen if the angular separation is close to 90°
 49
49The solution below is wrong! :( check later posts for right answer!
2) In my opinion b>a
that is because:
after departure of Cl in both, a primary carbocation is formed but in b after hydride shift a stabler carbocation is formed than in a..
thus, b>a
after all everything in organic chemistry drools for stability! [3]
 49
491) I look at this as a nucleophilic substitution..
If the nuleophile is stabler than the leaving group then the reaction is not feasible.
Now u know which is stabler and thus decide the answer! [6]
Basically mai bhool gaya hoon which is stabler CH3- or Ph- [3]
 1
1bhaiya but fajan sir told that in SN1 we generally do not consider hydride shift as soon as cl is gone that structure is seen for stability
 49
492) ohh uff! he is absolutely right! damn me! :(
sab bhool gaya sab!
a>b right answer!
reason more hyperconjugative structures!
 30
30Thanks evryone for all other questions. But for the question 1, my doubt was precisely the difference in stability of CH3- and Ph-. Agreed in Ph-, charge is not delocalised by resonance and faces repulsion from pi electron cloud, but the charge resides on a more electronegative carbon atom as compared to CH3-. Moreover, charge density on CH3- is also very high. In such a case, how do we decide? [7]
 49
49In Benzene the negative charge exists on the sp2 orbital and hence is concentrated on a single C not diffused.
In CH3 too the electron pair is concentrated on a single carbon on a sp3 orbital..
In CH3- the negative charge is borne by a less electronegative C than in Ph- ..
thus, Ph- is stabler!
P.S.=> The above solution is subject to market risks (based on mental logic and not experimental verification), please verify before trusting! [3][3]
 39
391) Either all of us have gone insane, or I'm wrong.
Ph- does not represent a resonance stabilized ion...
Spot the difference -:
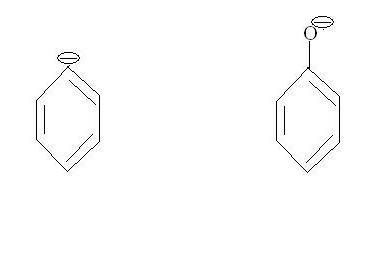
The left ion is Ph- and the right one is a resonance stabilized phenoxide ion (an example of resonance stabilized oxygen anion). Had it been a R-OPh, it would definitely have made a good leaving group.
The negative charge is perpendicular to the available resonance orbitals in Ph-...there can be no resonance.
Thus both the methynide anion and this anion are unstable, and that substitution is not at all feasible. Have you ever seen a directly attached unsubstituted benzene act as a leaving group??
Edit : Didn't see subho's last post. Indeed the charge is concentrated and not diffused, but why on earth would benzene act as a leaving group without any stabilizing/helper factors?
 30
30Exactly. So even though Ph- appears to be slightly more stable than CH3- as pointed out by Subho da, yet this reaction is not feasible right?
 71
71Pka for Benzene ~ 43
Pka for CH4 ~ 60
 39
39Yes, I believe that this reaction is not feasible. Besides, the methynide anion is a very strong base and would rather gain a proton than ever participate in nucleophilic substitution.
 49
49What if the solvent is aprotic @Pritish?