Like subho explained, http://www.chemguide.co.uk/organicprops/arenes/fc.html
Check out Friedel-Crafts' Reactions of methylbenzene here. The product ratio is temperature dependent.
Two doubts:
Why does methylation of toluene in the presence of anh. AlCl3 give m-xyelene as opposed to p- or o- product?
When 2 Bromo 1 Methyl cyclohexane is treated with CH3ONa in methanol solvent, does the reaction proceed with SN1 or SN2 and why?
-
UP 0 DOWN 0 0 10

10 Answers
for the first one...!
i am not remembering exactly but i think i had read something about the formation of the ortho and para substituted products but them getting transformed to meta...
some sort of equilibrium playing action!!
will have to think on it a bit..will get back with that soon...! very soon..!
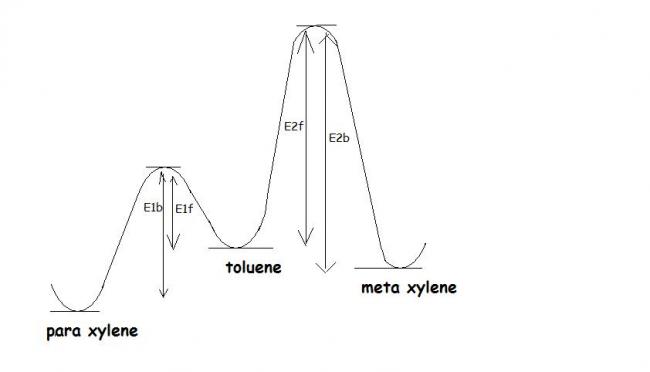
the E act of rxn from toluene to p-xylene is much less than toluene to m-xylene and thus initially p/o-xylene is formed in giant ratio as compared to m-xylene.
but in case of m-xylene the reverse E act is higher than that for o/p-xylene.
thus at especially elevated temperature a lot of o/p-xylene re forms toluene but tht is not possible for m-xylene...
the process continues and the molecules get "accumulated" towards the m-xylene end separated by large mountain of E act.!
in figure...(E1 is energy related to rxn toluene = o/p-xylene & E2 for the other one..f signifies forward rxn ; b signifies backward rxn)
btw was the pic i drew beautiful?? :D :D :D
second one i am not quite sure of...please validate it with some expert..! :D
In the chair (most stable) conformation of cyclohexane there are 2 types of bond possible axial and equitorial..!
suppose bromine is in equitorial position..!
Back side attack not possible since the nucleophile cannot attack from "within the ring".
so in this case there will be NO SN2 and there will be only SN1..!
again if it is present in axial position....back side attack not possible again due to crowding on the other side by 3 axially bonded atoms..! Again no SN2 and thus SN1.
Hence there will be ONLY SN1!! :D
DISCLAIMER:: Absolutely unsure..! Seek validation from some expert! [1]
what abt the second one? plz validate/reject! :-s
@sword: koi known answer hai kya re??