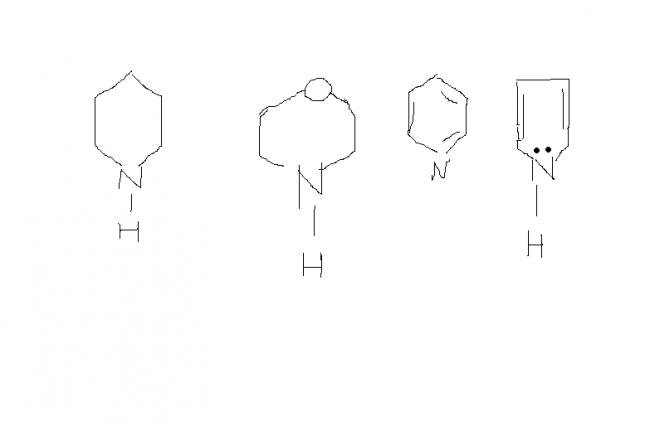
is the ans 3>1>2>4
7 Answers
kamalendu ghosh
·2009-04-02 07:20:03
In 4 delocalisation of electron on N takes place......in 3 delocalisation of e- does not take place (for mainting aromaticity)...so 3>4
In 2 O is e-withdraing so e over N decreases....In 1 no such takes place..
So 1>2>3>4
skygirl
·2009-04-02 19:29:22
yep 1>2>3>4 :)
@richa... the pyrine carbons are sp2 hybridised... more electronegative... they attracts the lone pairs...