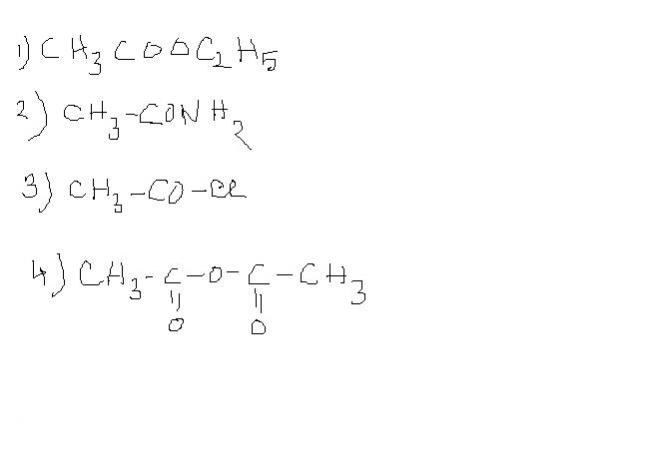not funny.serious.......change pic yaar put ur clear photo[3][3][3].....
36 Answers
yep... ofcrs..
OEt is better leaving grp than NH2-.. shak tha?
all that matters here in this question is the diff in leaving groups ..
haan mujhe yaad nahi tha.... wo to tukka tha mera answer between
4312 and 4321
@matrix... yaha q discuss kar rahe hai sab... aur tu mera photo discuss kar raha hai.. [3]
well i know 4 and 3 hav great compition but i think 3 wins

do correct me
OMYGOD!!! I DID A BLUNDER.....
IT IS 3>4>1>2 ......
ACTUALLY COPY ME JIS ORDER PE Q LIKHA THA.. MAIN US ORDER MEIN NAHI LIKHI YAHAN PE.... ISILIYE ALL THIS...
SOORY ABHISHEK U WERE WRONG....
AND SORRY VISHAL U WRE RIGHT..
PHY KARTE WAQT CHEM KARNE SE YAHI HOTA.........
isiliye maine poocha tha... acidic ya basic...
in acidic 4>3
ain basic 3>4
because in 4 in acidic medium it protonates ....
pata nahi ye bhi galat hi hua hoga... :(
mera aaj dimmag thik nahi hai.. :'(
look at the conjugate acids :
HCl and EtOH......ethyl alcohol is a weak acid and hence has a stronger conjugate base than hydrochloric acid !....we know that leaving tendency is inversely proportional to basicity....hence OEt- is a sronger leaving group than Cl-
the reasons i think are that
1. Cl- has more polarisability than OEt-.
2. CH3CH2------ gp is bulky hence it inc the basic strenghth of EtO-
ek question yaad aya:
arrange in inc order of hydrolysis..
1) CH3-COO-Cl
2) CH3-COO-OEt
3) CH3-COO-Me
4) CH3-COO-NH2