try
why cyclohexene reacts with K liquid NH3 gives cyclohexane giving poor yield????????
-
UP 0 DOWN 0 0 8

8 Answers
Abhishek Priyam
·2009-02-21 00:25:02
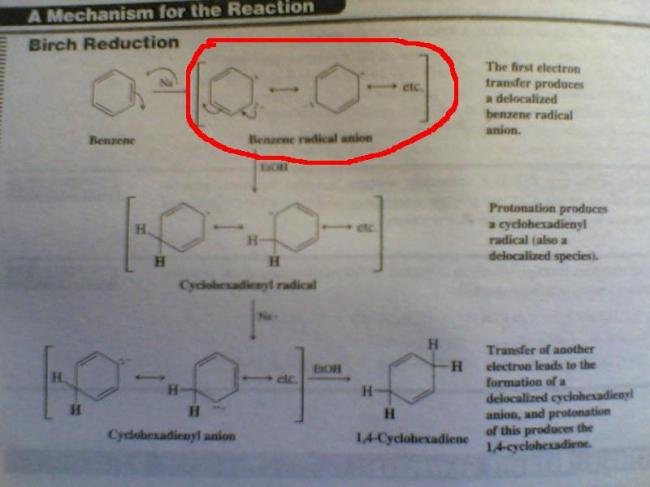
In cyclohexene theres no such resonance which stabilises the radical.. so initital transfer of electron from K is not so preferred...
Dr.House
·2009-02-21 00:26:30
priyam , then i think it should be applicable to all cases where no resonance is possible na.........???????????
Abhishek Priyam
·2009-02-21 00:27:40
yes...
in cyclohexene.. only 20 radical is formed... hence less stable..