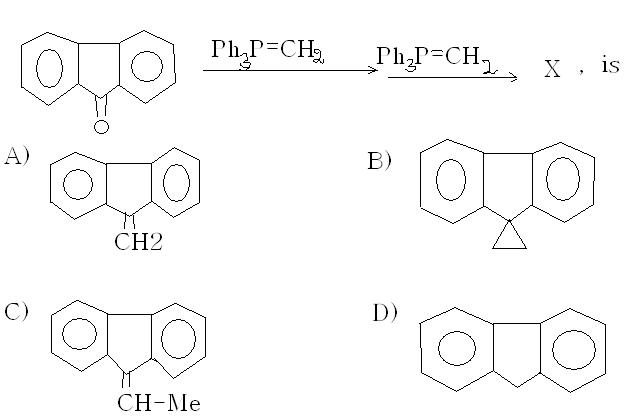
I think ans is A)....alkenes don't react with phosphorus ylides, not any reaction I've heard of. The first step is a Wittig reaction, and it gives a =CH2 in place of =O.
15 Answers
But that would be the case if the second reagent were a carbene...Wittig's reagents are not considered carbenes.
Pleaseee is ko solve kardo I konw it is wittig reaction but can't help myself here.
yes, the answer looks to be (b)
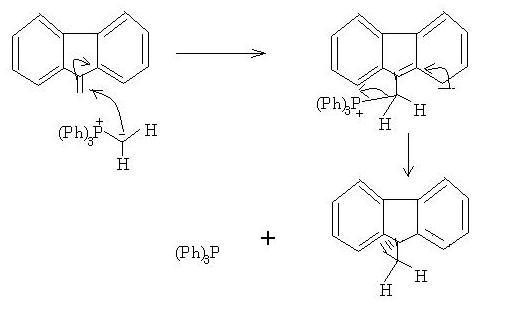
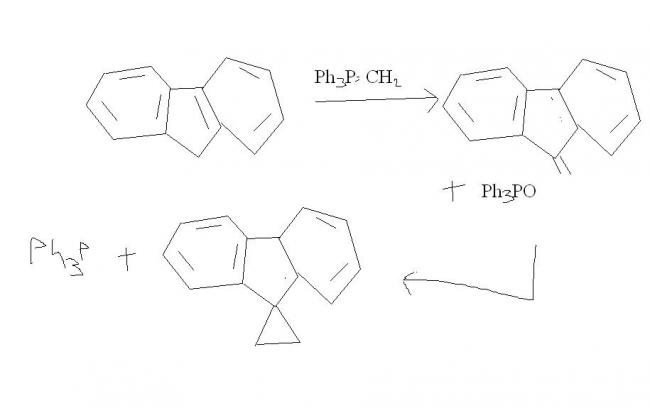
thats becasue in WITTIG , the decomposition of betaine is the rds ! after the first wittig, then the carbanion opens on the side of benzylic carbon of double bond ! after that there is a "hera-pheri" of bonds and we get the cyclopropane ring !
bolne se thodehi ho jayega.Please mechanism karke samjhao Debotosh.
hey iska mechanism mujhe nahin aata hai.pleasssse koi to batao.
Reopening the debate here :
I did a little research on this and found that the alkene acts as a Michael acceptor here(refer Michael additions).

The group on the top left with the EWG can be taken as the phosphorus ylide. Note the difference though; X is either S, P, or N. There is no EWG present(H can't really be an EWG) but phosphorus ylides are stable, so absence of EWG doesn't matter.
Now here's the catch; notice how bad the leaving ability of phosphorus is. If phosphorus doesn't leave, cyclopropanation nahi ho sakta...
This is what makes up for the catch - the three benzene brothers. Triphenyl phosphine is formed as side product, as -P(Ph)3 leaves and cyclopropanation does take place.
Will come up with a mechanism-diagram for this later.
And if you were wondering how this can happen without C=O, in Manmay's question there are two benzenes side by side which facilitate electron movement by resonance(like in C=O). This is the "opening on the benzylic side of the double bond" debotosh was talking about.