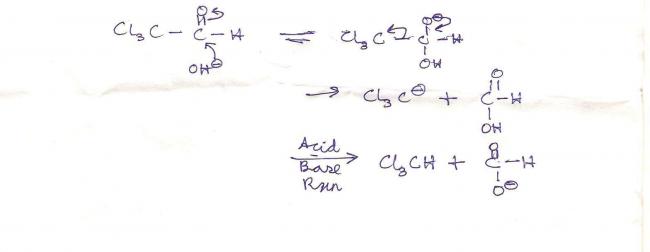
Indeed, CCl3- is a good leaving group due to the -I effect of the chlorine groups. Thus it leaves in place of the hydride ion as shown by organic. The last step in the rxn is similar to the last step of the haloform reaction.
actually this is a peculiar case in canizzaro reaction! the second compound does give canizzaro reaction but it is actually a kind of disproportionation !
I.L.FINAR says " although the canizzaro reaction is characteristic of aldehydes without α hydrogens, it is not confined the them . certain aliphatic α monoalkylated aldehydes undergo quantitative disproportionation when heated in aq NaOH at 170-200°C"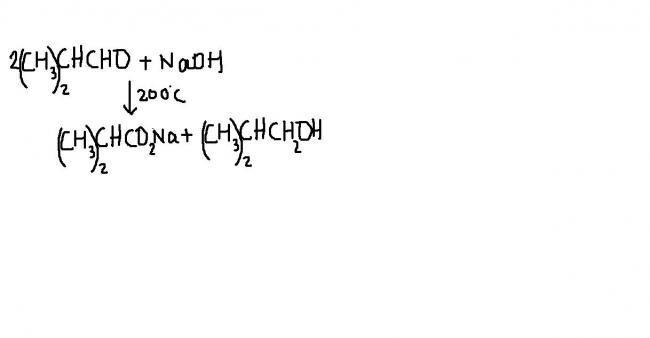
Indeed, CCl3- is a good leaving group due to the -I effect of the chlorine groups. Thus it leaves in place of the hydride ion as shown by organic. The last step in the rxn is similar to the last step of the haloform reaction.

there will be no canizzaro reaction in the first compound(i wrongly supposed the chlorine atoms to be hydrogen!) ! there is abnormal bond cleavage in the second sterp that inhibits canizzaro in the compound!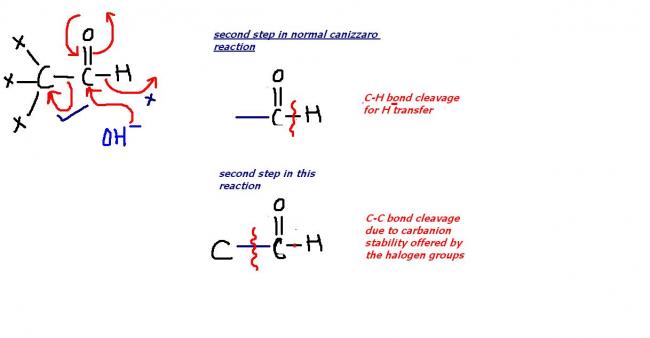
(b) will be giving cannizro reaction ..as an exception.
it's due to the reason that due to +I effect of the two alkyl groups . the carbanions of these aldehydes are not stable and hence do not undergo aldol condensation. Instead these aldehydes prefer to undergo nucleophilic attack by OH- ion at the aldehyde group giving an intermediate which acts as a hydride donor to the second molecule of the aldehyde leading to the formation of dispropornation products .
the rxn is as told by organic above !
the second one is a classical example where cannizaro wont occur...dont knw the exact reason but mah teacher told me to remember this spefic one!!
the first compound is a haloform reagent guys....so no chance of cannizaro...as CCl3- is a very good leaving group so H- cannot leave............nor will B give cannizaro as it has alpha acidic hydrogen n will prefer aldol!!
hey asish ... u were rite !!
(a) will not give
and (b) WILL give cannizaro reaction .
it's an exceptional case as u said ! just came across it !
Yeah, BT is right.. This question came in my Phase Test and I remember I did it wrong. CCl3CHO will not give cannizaro.
hmm celes was also telling that ans wud be (a)
reason given in BT was that the carbon is too hindered for the base to remove the acidic hydrogen while in (a) the I- undergoes subs with Cl which then results in formation of CHI3
BT has written in big bold words that it is an exception
about chloral , it will NOT give !
http://www.goiit.com/posts/list/organic-chemistry-does-chloral-give-cannizzaro-reaction-982362.htm