no.. this is also a carbene :CCl2 all that is required for a carbene is a lone pair
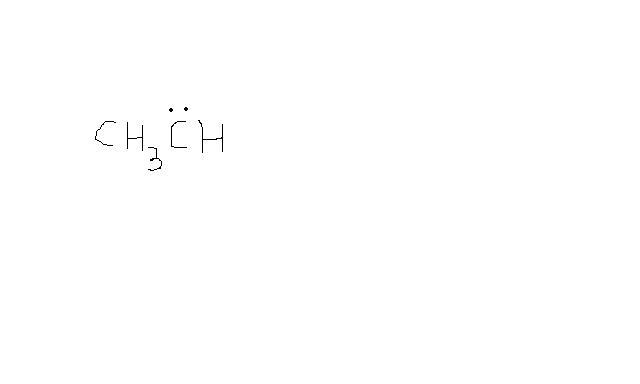 Is this a singlet carbene???
Is this a singlet carbene???
I think it shld have 2 hydrogen .........
-
UP 0 DOWN 0 0 6

6 Answers
Asish Mahapatra
·2010-06-12 23:30:03
the carbon which has lone pairs has already made 2 bonds. Hence there is no need for any extra H. If you are drawing a carbene, then the image is correct
euracle
·2010-06-12 23:35:52
Isn't it necessary that for a carbene both the bonds shld. be with hydrogen and none else??
Asish Mahapatra
·2010-06-12 23:36:57
Pritish Chakraborty
·2010-06-12 23:55:00
Asish is correct...infact there are carbene-like intermediates also, such as nitrenes.