dats y .... d>a>c>b
 benzene having a 3member ring on adding ch2n2 get converted to d benzene having 4 member ring
benzene having a 3member ring on adding ch2n2 get converted to d benzene having 4 member ring
-
UP 0 DOWN 0 0 55

55 Answers
my ans is correct n i m having soln but i m not able to understand it. if anyone interested in describing it then i ll post the soln.
my ans is correct n i m having d soln but i m not understand it.If anyone interested in describing it then i ll be post d soln.
hey in (b) how can double bond go to last-Ph
mera wala photo dekho naa...
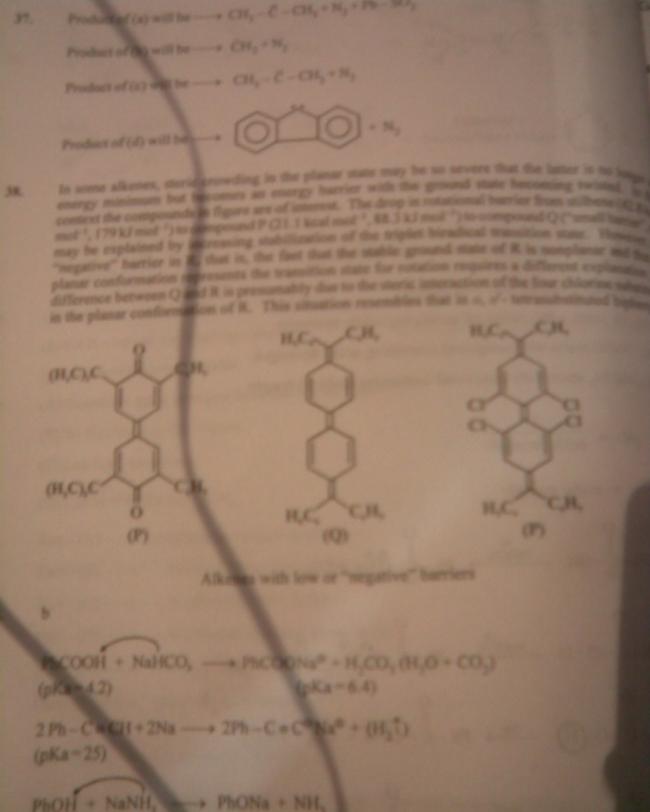
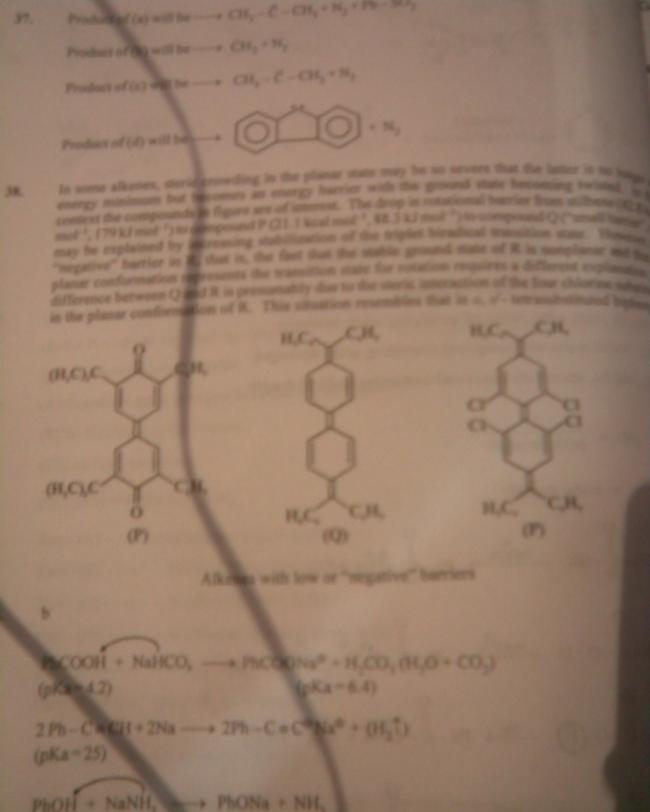
in some alkenes ,steric crowding in the planar state may be so severe that latter is no longer an energy minimum but becomes anenergy barrier with ground state becoming twisted .in this context ,compounds in fig. are of interest .d drop in rotational barrier from stilbene [42.8kcal mol-1,179KJ Mol-1] to compound P[21.1Kcal mol-1,88.3KJ Mol-1] to compound Q
[small barrier e.f.] may be explained by increasing stabilization of triplet biredical transition state.however -ve barrier in R, i.e.fact that planar conformation represents transitionstate for rotation require a different explanation; diff.bet. Q n R is presumably due to steric interaction of 4 Cl substituents in polar conformation of R. this situation resembles that in O,O'-tetrasubstituted biphenyls to
:O
sorry i can't help... mujhe samjh me nahi aa raha...
is it in english :P
richa.. try using a jpg image instead of a bmp.
you can "FILE -> save image as " on Paint to do this.