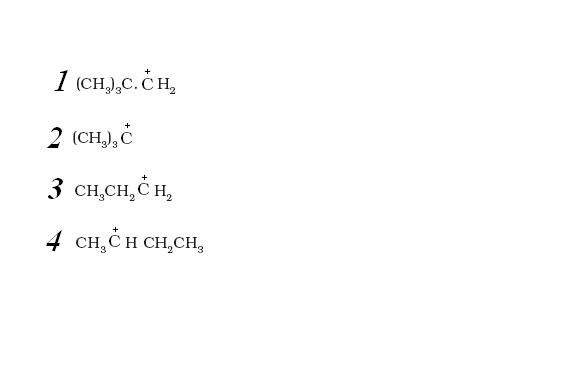1
1BUDDY.WHEN DID I SAY THERE R THREE BONDS.................I SAID
now even if you look at 3 and 1 from inductive effect point of view,the inductive effect in both should be nearly equal(coz although one has three methyl groups for inductive effect but they r at a distance of two bonds).so,inductive effect in 1 and 3 is nearly same.
MOREOVER, Y R U NEGLECTING THIS
in addition to inductive effect there is hyperconjugation in 3.
so clearly 3>1.
 1
1what 3 bonds ???/
there's only one bond
here I takes more importance
and b/w questioing is your right
without questions nothign would have been discovered or invented
 1
1N IF MY REASONS R WRONG........THEN WHY???????????
[PLZ DON THINK I M ARGIUNG!!!!!! AS U ONLY SAID WE SHUD HAVE CLEAR CONCEPTS.........THAT'S Y ALONG WITH CORRECT REASON U TOLD IT IS NECCESARY TO KNOW Y MY REASONS R WRONG]
 1
1SRINATH DA.......I DON QUESTION UR ANS. BUT I WANNA KNOW ISN'T REASON I GAVE IN #45 EQUALLY CORRECT!!!!!!!!!!!!!!
 1
1see as far I think my answer seems quite correct ,maybe wrong but 99% sure not 100%
try to go thru Sykes .
hopefully you'll get it .
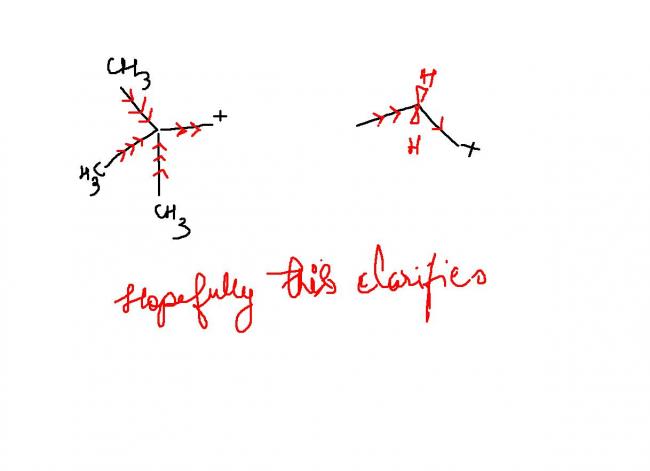
 1
1srinath da...........plz see this:
see i think 3>1
REASON:inductive effect vanishes after three bonds.........
now even if you look at 3 and 1 from inductive effect point of view,the inductive effect in both should be nearly equal(coz although one has three methyl groups for inductive effect but they r at a distance of two bonds).so,inductive effect in 1 and 3 is nearly same.
now,in addition to inductive effect there is hyperconjugation in 3.
so clearly 3>1.
 1
12>4>1>3
here inductive effect takes over I guess . see there is also what we call as the space effect (I dunno whether it's the right term .but I guess it vaguely tells what I mean )
effects transmiteed thru spcae . this acts here which reinforces the Inductive effect
this is mostly given in Peter SykesI guess go there for further studying :)
he discusses space effect ( fogive me for this name )
I think you should read it from there for better understanding
 1
1mera toh 2>4>3>1 hi a raha hain
 1
1hey man
u c in 1 there is a tendency of METHYL shift & hence it is wanting to go to a more stable state 4m an unstbl state............
hence we can expct that 1 is least stable
so John i think ur corrrrrrr...........
if anyone has the guts to talk against we 2 den plz cal me at 100......
 1
1SRINATH DA U ONLY TELL WAT IS CORRECT ORDER
 1
1kool......den i might b wrong[7]
 1
1@archana
read #20 in this thread....n then tell wat do u think?????????
 1
1itz 2>4>1>3 ...because alkyl group are electron donatin,therefore more the number of alkyl more will be the stability of carbocation .....so 1>3
 1
1der are 2 factors...e-cloud push by 3 Me groups, 2 alpha Hydrogens giving rise to HCE...i think 3 must be more stable than 1...
 1
12>4>3>1
3,1 are compared on the basis of hyperconjugation and u can think of the rate tat in 1 is highly unstable and wants 2 change via CH3- shift but in 3 hydride shift is difficult
 1
1srinath bro............plz reply
 1
1guys check these out .
they aer from sykes
go thru I guess there should not be any doubt
he's given for -I I've extended for +I that's all
. hopefullly I'm right . still the hyperconjugation one is in doubt according to me and I stick to my original ans


 11
11areey
anand
seedhi si baat hai
1>3
REASON:(CH3)3will pose more hindrance for the attack compared to CH3CH2
 1
1REASON:(CH3)3will pose more hindrance for the attack compared to CH3CH2
MANIPAL
WE ARE TALKING BOUT STABILITY NOT REACTIVITY
 1
1ur answer is perfectly rit dude......c the no. of alpha hydrogen...n u will get that u r rit...be confident bro...
 9
92>4>1>3
i agree with skys ans
JC i saw ur post 20
but i dont agree with ur exp
reason :
inductive effect is more in 1
inductive effect is more dominant than hyperconjugation (no bond resonance)
 1
1ARE YOU SURE CELESTINE...............HYPERCONJUGATION IS INSIGNIFIANT???????
 1
1@celestine and srinath
hyperconjugation is after all """no bond resonance"""" and more specifically an extension of """""""resonance effect or mesomeric effect""""""""""""....................so it shud get some credit in deciding stability.....................isn't it!!!!!!!
 1
1I think so the correct answer is
2>1>4>3
because when the carbocation formed is more stable then it is more reactive. or in other words when any carbon compound is more reactive it will convert to its carbocation form & it must be stable because any compound will not transform into unstable form.