2>4 >3>1
ARRANGE FOLLOWING CARBOCATRION IN DECREASING ORDER OF STABILITY
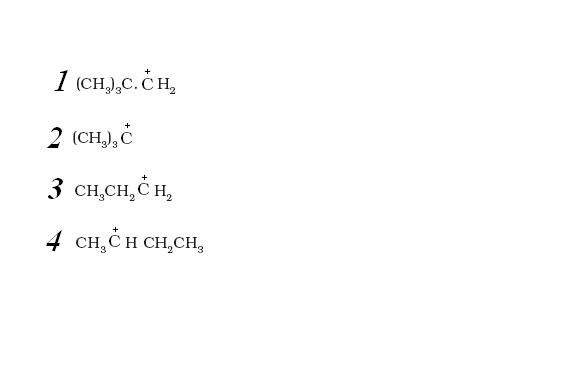
I THINK IT SHUD BE 2>4>3>1
AM I CORRECT?????
-
UP 0 DOWN 0 1 65

65 Answers
@pranika
did u find this answer in some book of urs.............??????
@ANIRUDH AND METAL
AS FAR AS I THINK IT WUD BE 3>1 COZ OF HYPERCONJUGATION IN 3
......DO U AGREE?????????
bhai bhai, i don't know any complex terms like hyperconjugation and all....but i think 1>3 because, even though the catbocation itself is not tertiary, it can become more stable by means of a methyl shift.
It is probable-------- I had thought of inductive effects only,
I'll have to rethink--------
[ Isiliye mujhe Organic Chemistry pasand nahin-------- kuchh bhi ho sakta hai :-( ]
i only thought of inductive effect while posting the first reply....but now also thinking interms of methyl and hydride shifts
Anirudh, by methyl shift, 1 will change to a 2ndary carbocation.
Similarly, 3 can also change to a 2ndary carbocation by hydride shift.
yes, that's why i am reluctant...
maybe 3 will win over 1 because of lesser steric hinderance!!
are yaar...y r u thinking of methyl shift etc.
i simply mean to say
1.that in 3 we will have hyperconjugation as C-H bond is adjacent to positively charged carbon.this will disperse positive charge on carbocation and 3 attains stability.
2.whereas in 1no such hyperconjugation is possible as there is no C-H bond adjacent to positively charged.
so due to above two reasons i think 3>1
hope after reading above two reasons u agree with me!
so which will win over the other?
HYPERCONJUGATION vs INDUCTIVE EFFECT
VENUE: http://targetiit.com/iit_jee_forum/posts/carbocation_stability_8716.html
MATCH ORGANISER: JOHN CENA
MATCH REFREE: ANI
WINNER??
see i think 3>1
REASON:inductive effect vanishes after three bonds.........
now even if you look at 3 and 1 from inductive effect point of view,the inductive effect in both should be nearly equal(coz although one has three methyl groups for inductive effect but they r at a distance of two bonds).so,inductive effect in 1 and 3 is nearly same.
now,in addition to inductive effect there is hyperconjugation in 3.
so clearly 3>1.
hey skygirl plz check ma explanation????????
yahi toh organic chem ka kharabi hai ... ki u have to use the correct concept at correct place ... which even my sirs are not able to do .. so chill out .. don't waste ur time in this question !
Kya koi iska definite answer de sakega-------- after all, it's ORGANIC CHEMISTRY------- kuchh bhi ho sakta hai.
n i think my xplanation in #20 is correct.so,definite ans. seems to be
3>1.
if i m wrong correct me!!!!!!!