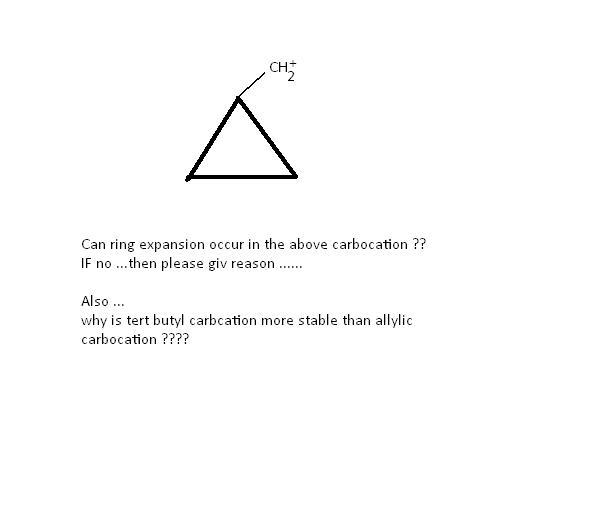
1
1ring expansion can occur
carbon in the cyclopropane has sp3 hybridisation(shld have 109.5 degree bond angle)..but actually it is only 60 degree. So ,to relieve angle strain,ring expands to form a 4 carbon ring ,cyclobutane(90 degree bond angle)!!
2)) tert butyl more stable because of 9 hypercojugative structure.An exception where hyperconjugation dominates over resonance stablisation.
9 hyperconjugation > resonance
 13
13Ring expansion wud occur as Cyclo Butane is stabler than a cyclo propane......
n fr the 2nd Hyperconj. is given preference over allylic Resonance.....
 106
1061. instead of ring expansion a viable option is there if u consider its hyperconjugative structure which is free of any sort of strain which was posted by mahato in one of my doubts
here's the link:
http://targetiit.com/iit-jee-forum/posts/complete-the-reactions-11185.html
2. tert butyl carbocation has 9 Hyper Hydrogens which overpowers the sole contribution of resonance in allylic as well as benzyllic carbocations
 23
23So wat shud i deduce ...ring expansion occurs or not ?? to form a 90degree free of strain secondary C+ OR no ring expansion ???
 11
11NO RING EXPANSION WONT OCCUR..
tHE reactant is more stable than even the following compunds...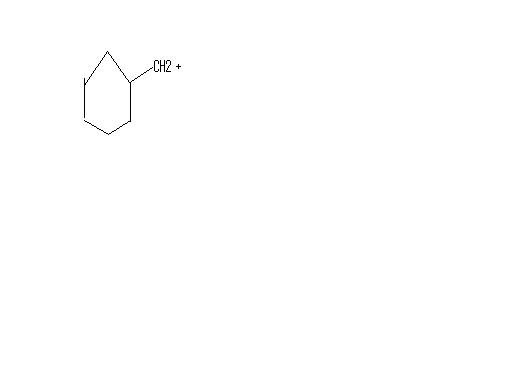
&
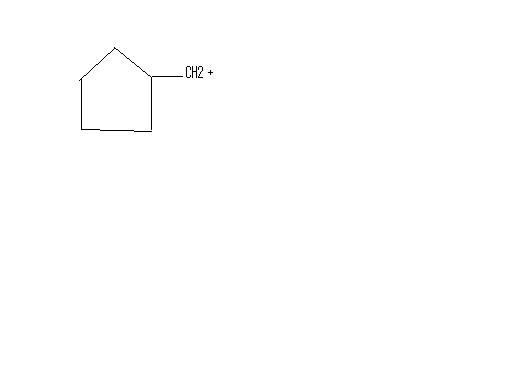
this is because the positive charge is delocalized in the p-orbitals...draw the p-orbitals of the 4 carbons..ul c how.
 11
11sorry for the space..dont know how to decrease it..
 23
23kk tnx ..i expected dat answer....jus wanted to know the reason for it
 39
39The cyclopropane ring is under high angle strain, packing those bonded electrons under 60° angles. Having an electron deficient adjacent carbon helps relieve some of the strain on the ring.
 23
23"Having an electron deficient adjacent carbon helps relieve some of the strain on the ring."How???
abhi bataya hai to explain karna hi padega lolz [6]
 39
39p orbital delocalisation? the carbocation craves electrons much like you crave pizza :P
 23
23are u talking abt hyperconjugation ???? lolz aisa bol na ...itna complex me mat bata ....main tere jaisa organic ka master nahi hai ...lolz [3]