it is named as D because the carbon atom attached to the lowest chiral carbon has the OH group to its right (dextro rotatory)
and that's the general rule
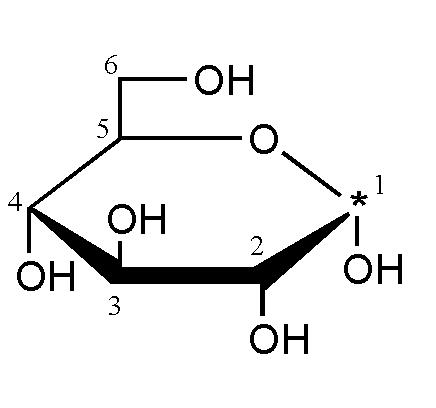
 Why diz compund is named as α-D-glucopyranose.
Why diz compund is named as α-D-glucopyranose.
i just want to know that, what is the significance of 'D' ?
-
UP 0 DOWN 0 0 13

13 Answers
All compounds having the arrangement similar to (+) - Glyceraldehyde are called 'D' while the other 'L'. ( the last C in case of Glucose )
CHO
|
H-C-OH
|
CH2OH
(+) - Glyceraldehyde
CHO
|
OH-C-H
|
CH2OH
(-) - Glyceraldehyde
The structure of α-D-glucose is
-----------
| |
H-C-OH |
| |
H-C-OH |
| |
OH-C-H O
| |
H-C-OH |
| |
H-C---------
|
CH2OH
Which is got from
O
||
H-C
|
H-C-OH
|
OH-C-H
|
H-C-OH
|
H-C-OH ---> is similar to (+) - Glyceraldehyde
|
CH2OH
Therefore it is called α-'D'-glucopyranose.
It is not 'D' because it is dextrorotatory ....
It is shown if it is dextrorotatory by putting the sign (+)
considering the structure i have posted, how can we say that OH attached is at right.... may be i m confused
In the structure you posted, the OH has added with the CHO group..and H is on the left .. so OH must have been on the right.. the open chain and ring structure exist in equilibrium..
varun you explained good but i am not getting. rohan said that carbon atom attached to the lowest chiral carbon is having the OH group to its right carbon atom attached to the lowest chiral carbon is marked 5 but it does not have any OH. Rather it has CH2OH & H.
check this out , it's good , read only the D/L part and tell.
http://en.wikipedia.org/wiki/D-form#By_configuration:_D-_and_L-
so aman with this fig. u can apply d concepts which others have given.. and u r right D doesnt mean its dextro or something... Its just given on the basis of the presence of OH bond on glyceraldehyde..........
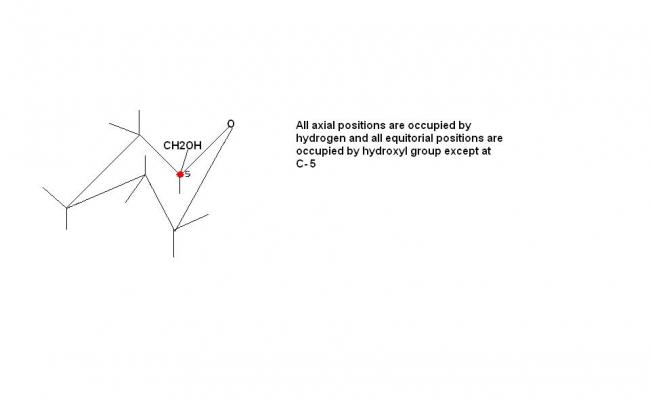
but OH group at 1 & 4 are above the plane becoz equitorial bond is above the plane